Ackerman bacteria Amuc2172 protein as well as preparation method and application thereof
A protein and application technology, applied in the field of biomedicine, can solve problems such as intestinal inflammation and poor cancer treatment effect, and achieve the effects of easy commercialization, preparation and promotion, high drug efficacy, and avoiding a single route of administration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
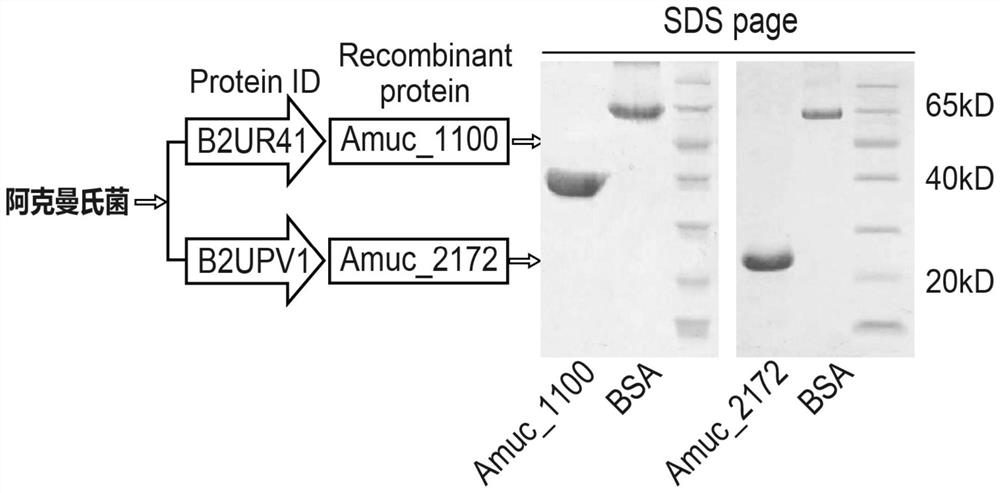
[0038] Akkermansia protein purification: clone Akkermansia Amuc_2172 gene (see https: / / www.ncbi.nlm.nih.gov / nuccore / CP001071.1?from=2650234&to=2650734) (gene sequence is: atgccgactc tccgccaagc cgccaaggac gacatcacgc tgatccatga actggcctgccaggcttttc cggccacata ccgggacctc ctttccagag agcagataga tttcatgatg gactggatgtattcccccgc caacctggaa aaacagatgg aggaggggca cgtgtacttc atcgcctccc atgagggaaaggactgcggc tacctgtccg tccagccgga aggccccggc gtcttccatt tgcagaaaat ctacgttctgcccggcttcc agggcctgca tataggaagc tttctgttcc gccacgccat cagctacatc agaagcatccacccggaacc gtgcctgatg cgcctgaacg tgaaccgcga taacacccgg gcggtggaat tctaccagcgcatgggcatg cggaccctgg aacgcgggga tttccacatc ggccacggtt actacatgac ggactacatcatgggactgg atatagcctg a)(SEQ ID NO.2 shown) and the Amuc_1100 gene (see https: / / www.ncbi.nlm.nih.gov / nuccore / CP001071.1?from=1314411&to=1315364) (the gene sequence is: ttaatcttca gacggttcct gagccttggg ctggttgaag tggaccagat tcagggagacctggacag gacctggtggctcctggctgcatg tgcaggtgcagccgcctcat ccgccggagt c...
Embodiment 2
[0042] Example 2 evaluates the effect of Amuc_2172 on Th17:
[0043]Wild-type C57BL / 6J (8-10 weeks old) mice were taken, killed by neck pulling, and spleens were taken under aseptic conditions. In a beaker filled with 10ml of 4°C normal saline, cut it into pieces with ophthalmic scissors, gently grind it through a 200-mesh nylon mesh, and then sieve it to collect the cell suspension. Centrifuge the suspension at 600×g for 5 min. Discard the supernatant, observe the volume of the precipitated cells, add red blood cell lysate at a volume ratio of 10:1, mix well and let it stand for 2 minutes, then immediately add 10ml of 4°C normal saline, centrifuge at 600×g for 5 minutes. The supernatant was discarded, and the cells were repeatedly washed twice with 4°C saline. Carry out cell count before the last centrifugation, and resuspend cells with RPMI-1640 complete medium (the RPMI-1640 that contains volume percent concentration is 10% fetal bovine serum adds penicillin and streptomy...
Embodiment 3
[0045] Example 3 Safety Assessment of Amuc_2172 Different Administration Methods:
[0046] Wild-type C57BL / 6J (8-16 weeks old) mice were taken, and no more than 5 mice were raised in each cage. The mice were marked and weighed for their reference body weight. Thirty mice were randomly divided into three groups. In the control group (Ctrl, Control group) animals, physiological saline was injected into the tail vein. In the injection group, 150 μg / kg body weight of Amuc_2172 protein was injected into the tail vein. The animals were injected from the 0th day, injected three times a week, observed the general condition of the animals every day, water intake, body weight, and stool properties.
[0047] The daily body weight loss percentage of the animal was calculated using the following formula: [(body weight-reference body weight) / reference body weight]×100. On the 10th day of feeding, the experiment ended and the animals were sacrificed. Evaluate the survival status and body ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com