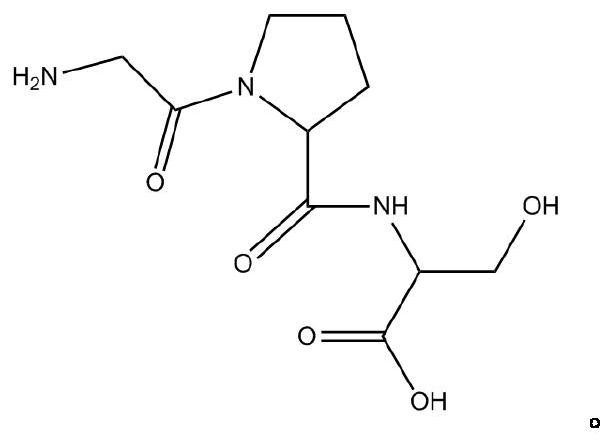
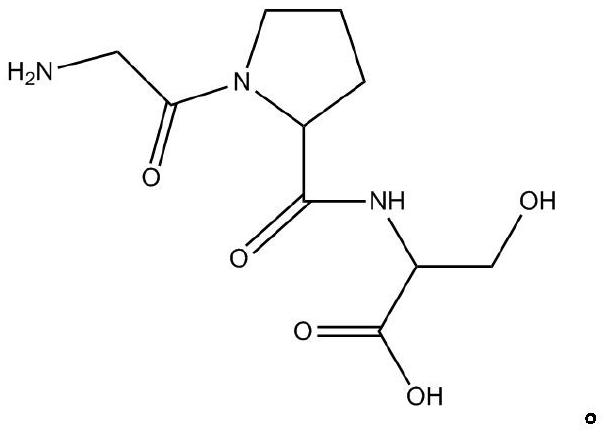
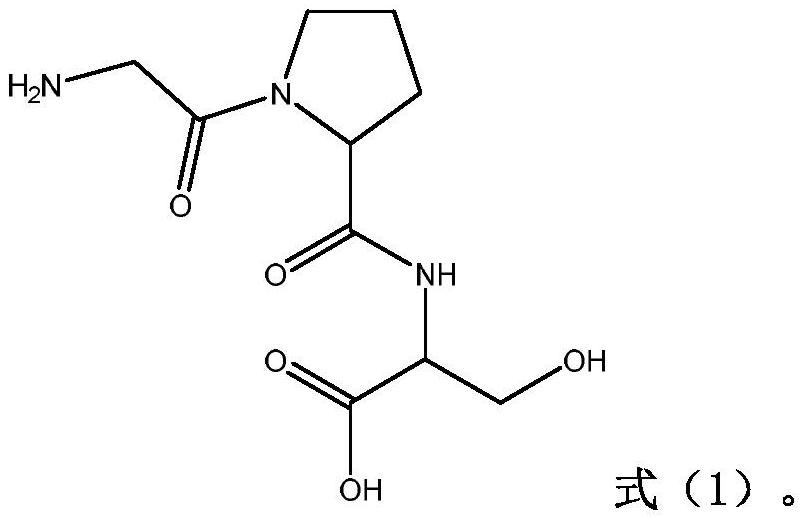
Pharmaceutical composition for preventing phlegm from forming and stopping coughing
A technology for reducing phlegm and relieving cough and composition, which is applied in the field of medicine and can solve problems such as physical exertion and destruction of alveolar wall elastic tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The preparation of embodiment 1 glycyl prolyl serine oral solution (unit: g)
[0084] Raw materials Prescription 1 Prescription 2 Prescription 3 Glycylprolylserine 2g 4g 8g Sorbitol 30g 30g 30g Potassium sorbate 0.15g 0.15g 0.15g Citric Acid Monohydrate 0.2g 0.2g 0.2g 0.1M hydrochloric acid / sodium hydroxide Appropriate amount Appropriate amount Appropriate amount Solution pH 6.5-7.5 6.5-7.5 6.5-7.5 Purified water to a volume of 100ml 100ml 100ml
[0085] .
[0086] illustrate:
[0087] According to the low, middle and high doses administered to the mice, it is converted into the adult dose according to the body weight (the adult body weight is 60kg, the conversion coefficient is 0.11, and each time taking 5ml oral liquid, each time taking about 0.1g, 0.2g or 0.4g)
[0088] Oral liquid preparation process is as follows:
[0089] 1) Take glycylprolylserine, sorbitol, potassium sorbate, citric...
Embodiment 2
[0094] The preparation of embodiment 2 capsules
[0095] Raw materials Prescription 1 Prescription 2 Prescription 3 Glycylprolylserine 100g 200g 400g microcrystalline cellulose 42g 84g 168g Mannitol 42g 84g 168g Croscarmellose Sodium 10g 20g 40g Micropowder silica gel 4g 8g 16g Sodium stearyl fumarate 2g 4g 8g Weight of the contents of the unit dosage capsule 200mg 400mg 800mg HPMC capsule shell 1000 capsules 1000 capsules 1000 capsules Co-made 1000 capsules 1000 capsules 1000 capsules
[0096] .
[0097] According to the low, medium and high doses given to mice, it is converted into adult dose according to body weight (adult body weight is 60kg, the conversion coefficient is 0.11, take one capsule each time, and take 0.1g, 0.2g or 0.4g each time)
[0098] Preparation Process:
[0099] 1) Take the prescription amount of glycylprolylserine, microcrystalline cellulose, mannitol,...
Embodiment 3
[0106] The preparation of embodiment 3 tablet
[0107]
[0108]
[0109] According to the low, middle and high doses administered to mice, it is converted into an adult dose according to the body weight (the adult body weight is 60kg, the conversion coefficient is 0.11, and one tablet is taken each time, 0.1g, 0.2g or 0.4g each time)
[0110] Preparation Process:
[0111] 1) Take the prescription amount of glycylprolylserine, microcrystalline cellulose, mannitol, pulverize, pass through a 80-mesh sieve, and set aside;
[0112] 2) Take the prescription amount of glycylprolylserine, microcrystalline cellulose, and mannitol, mix evenly, add an appropriate amount of purified water, and make a soft material;
[0113] 3) Take the soft material obtained in step 2), prepare wet granules with a 24-mesh sieve, dry at 60°C, and granulate with a 24-mesh sieve to obtain drug-loaded granules;
[0114] 4) Take the drug-loaded granules obtained in step 3), add croscarmellose sodium, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com