Synthesis method of deuterated albendazole
A technology of albendazole and a synthetic method, applied in the field of pharmaceutical preparation, can solve problems such as no literature reports, and achieve the effects of reducing production cost, improving atom economy, good reproducibility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of compound 1:
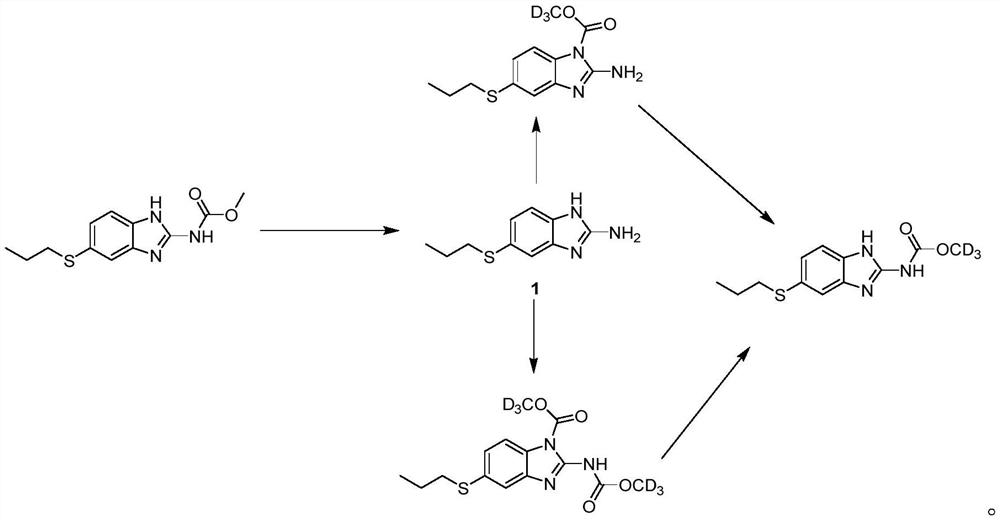
[0047] Add albendazole (8.0g, 30.2mmol), sodium hydroxide (1.8g, 45.0mmol), methanol (250ml) and water (50ml) in 500 milliliters of round bottom flasks, then reaction system heating reflux 16 hours, Bendazole raw material disappeared. Cooled to room temperature, concentrated under reduced pressure to remove most of the methanol, a large amount of solid precipitated, filtered, washed with water and dichloromethane in turn, the solid was dried to obtain compound 1, 5.5g, yield 88%, MS (m / z): 208.1[ M+H] + .
Embodiment 2-1
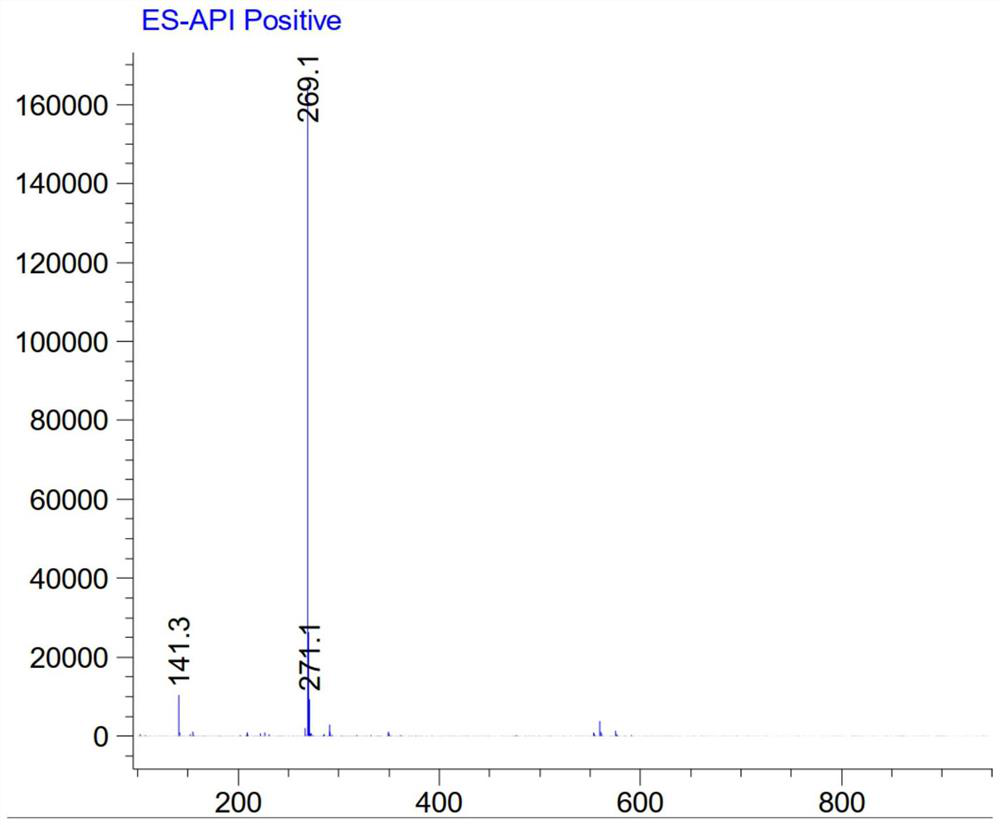
[0049] In a 50ml round bottom bottle, compound 1 (207mg, 1.0mmol), sodium bicarbonate (256mg, 3.0mmol) and acetone 6ml were added. With stirring, methyl deuterochloroformate (100 mg, 1.1 mmol) was slowly added. After the addition, the reaction mixture was stirred at room temperature, followed by TLC and LC-MS, and the reaction was complete within 2.5 hours. Filter, wash with water, dichloromethane and methanol successively, and dry the solid to obtain Intermediate 2, 220 mg, yield 83.0%, MS (m / z): 269.0 [M+H] + .
Embodiment 2-2
[0051] In a 100 ml round bottom bottle, compound 1 (2.1 g, 10.1 mmol), sodium bicarbonate (2.5 g, 30.0 mmol) and acetone 50 ml were added. Methyl deuterochloroformate (1.1 g, 11.2 mmol) was slowly added dropwise over 5 minutes. The reaction mixture was stirred at room temperature, followed by TLC and LC-MS, and the reaction was complete within 2.5 hours. Then heated to reflux for 16 hours, LC-MS followed the reaction, intermediate 2 disappeared, waited for the reaction to cool to room temperature, filtered, washed with water, dichloromethane and methanol successively, and the solid was dried to deuterated albendazole, 2.3g. Yield 85%, chemical purity 99.0%, isotopic abundance 99.5%, MS(m / z): 269.0[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com