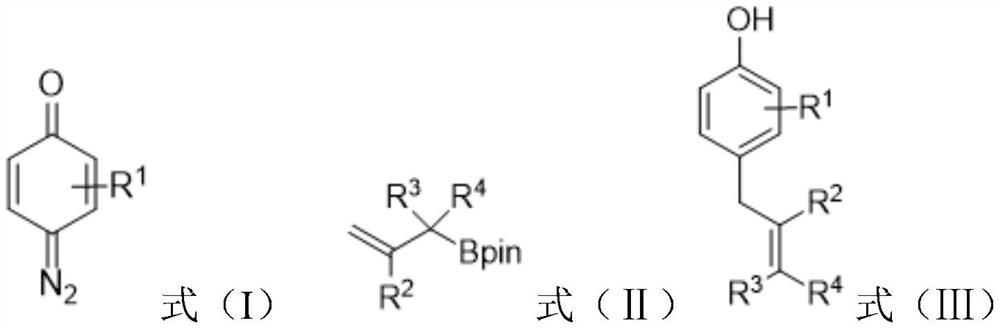
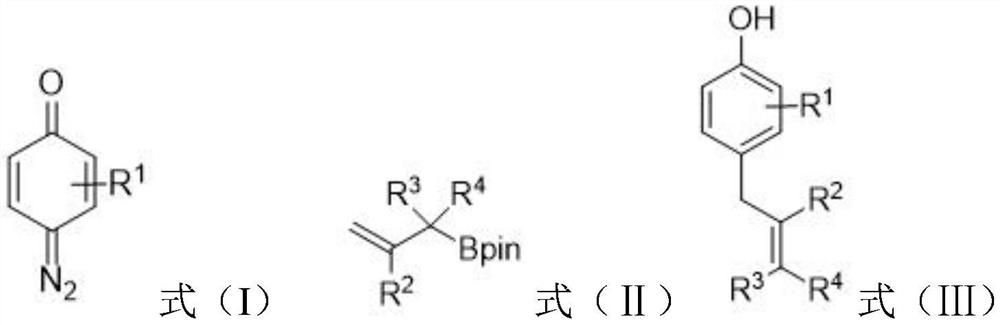
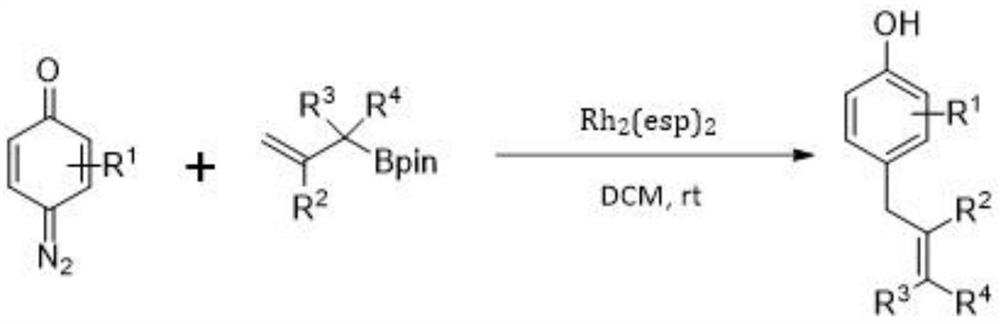
Preparation method of allyl phenol compound
A technology for allyl phenol and compound, which is applied in the field of preparation of allyl phenol compounds, can solve the problems of high reaction temperature, low selectivity, low yield and the like, and achieves the effects of reducing reaction temperature and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] This example provides a method for preparing 4-allyl-3-fluorophenol. In an argon atmosphere, add 4-diazo-3-fluorocyclohexyl-2,5-diene sequentially into a 10mL Schlenk tube -1-ketone (0.3mmol), Molecular sieves (60mg), then add anhydrous dichloromethane DCM (1.0mL), and stir for 1 minute, then add allylboronic acid pinacol ester (0.9mmol, 3.0 equivalents),
[0024] Rh 2 (esp) 2 (2mol%, 4.55mg) and dichloromethane (1.0mL). Then the above reaction mixture was stirred at room temperature until the complete consumption of the quinone diazide was monitored by TLC analysis. After the reaction was completed, the TLC plate detected that a product was generated.
[0025] Its reaction equation is as follows:
[0026]
[0027] After the reaction, the reaction mixture was filtered and washed with dichloromethane (20 mL), spin-dried, and the residue was separated through a silica gel column to obtain a pale yellow oily product with a yield of 94%. The NMR data of the product ...
Embodiment 2
[0029] This example provides a method for preparing 4-allyl-3-chlorophenol. In an argon atmosphere, add 4-diazo-3-chlorocyclohexyl-2,5-diene sequentially into a 10 mL Schlenk tube -1-ketone (0.3mmol), then add anhydrous dichloromethane DCM (2.0mL), and stir for 1 minute, then add allylboronic acid pinacol ester (0.9mmol, 3.0 equivalents), Rh 2 (esp) 2 (2mol%, 4.55mg) and dichloromethane (1.0mL). Then the above reaction mixture was stirred at room temperature and normal pressure until the quinone diazide was completely consumed by TLC analysis. After the reaction was completed, the TLC plate detected that a product was generated.
[0030] Its reaction equation is as follows:
[0031]
[0032] After the reaction, the reaction mixture was filtered and washed with dichloromethane (20 mL), spin-dried, and the residue was separated through a silica gel column to obtain a pale yellow oily product with a yield of 93%. The NMR data of the product are as follows: 1 H NMR (300MHz, ...
Embodiment 3
[0034] This example provides a method for preparing 4-allyl-3-bromophenol. In an argon atmosphere, 4-diazo-3-bromocyclohexyl-2,5-diene is sequentially added to a 10 mL Schlenk tube -1-ketone (0.3mmol), Molecular sieves (75mg), then add anhydrous dichloromethane DCM (1.0mL), and stir for 1 minute, then add allylboronic acid pinacol ester (0.9mmol, 3.0 equivalents), Rh 2 (esp) 2 (2mol%, 4.55mg) and dichloromethane (0.5mL). Then the above reaction mixture was stirred at room temperature and normal pressure until the quinone diazide was completely consumed by TLC analysis. After the reaction was completed, the TLC plate detected that a product was generated.
[0035] Its reaction equation is as follows:
[0036]
[0037]After the reaction was finished, the reaction mixture was filtered and washed with dichloromethane (20 mL), spin-dried, and the residue was separated through a silica gel column to obtain a pale yellow oily product with a yield of 85%. The NMR data of the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com