Preparation method of O-3-chloro-2-allyl hydroxylamine
A technology of propenyl hydroxylamine and O-3-, which is applied in the field of preparation of O-3-chloro-2-propenyl hydroxylamine, can solve the problems of low selectivity and cumbersome operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
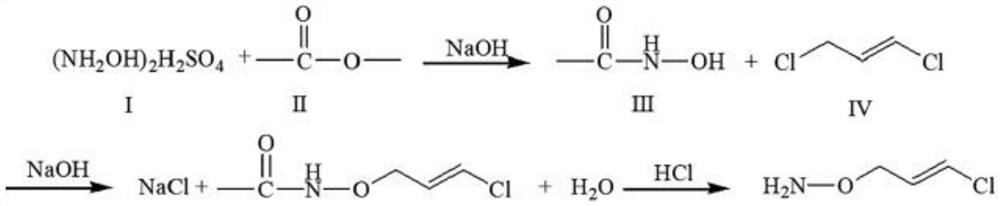
[0031] In the first three-necked flask, 71.0 g of distilled water and 100 g of hydroxylamine sulfate were successively added, and then 162.8 g of liquid caustic soda was added dropwise to prepare 207.2 g of hydroxylamine aqueous solution. Pour the prepared hydroxylamine aqueous solution into the second three-necked flask, then add 99.6g of methyl acetate, start stirring, then add 1.5g of 4-dimethylaminopyridine catalyst, and then dropwise add 196g of 30% NaOH aqueous solution (liquid Alkali), the temperature was kept at 20°C, and the reaction was carried out for 1 h. Add 136g of trans-1,3-dichloropropene into the second three-necked flask, raise the temperature to 50°C, and react for 7h. The molar ratio of the above raw materials n(hydroxylamine):n(methyl acetate):n(4-dimethylaminopyridine):n(NaOH):n(trans-1,3-dichloropropene) is 1:1.1:0.01 :2.1:1.
[0032] Subsequently, 180 g of hydrochloric acid was added, and after stirring, it was refluxed at 60° C. for 2 h. After the s...
Embodiment 2
[0034] In the first three-necked flask, 71.0 g of distilled water and 100 g of hydroxylamine sulfate were successively added, and then 162.8 g of liquid caustic soda was added dropwise to prepare 207.2 g of hydroxylamine aqueous solution. Pour the prepared hydroxylamine aqueous solution into the second three-necked flask, then add 99.6g of methyl acetate, start stirring, then add 15.0g of 4-dimethylaminopyridine catalyst, and then dropwise add 196g of 30% NaOH aqueous solution (liquid Alkali), the temperature was kept at 20°C, and the reaction was carried out for 1 h. Add 136g of trans-1,3-dichloropropene into the second three-necked flask, raise the temperature to 70°C, and react for 5h. The molar ratio of the above raw materials n(hydroxylamine):n(methyl acetate):n(4-dimethylaminopyridine):n(NaOH):n(trans-1,3-dichloropropene) is 1:1.1:0.1 :2.1:1.
[0035] Subsequently, 180 g of hydrochloric acid was added, and after stirring, it was refluxed at 60° C. for 2 h. After the s...
Embodiment 3
[0037] In the first three-necked flask, 71.0 g of distilled water and 100 g of hydroxylamine sulfate were successively added, and then 162.8 g of liquid caustic soda was added dropwise to prepare 207.2 g of hydroxylamine aqueous solution. Pour the prepared hydroxylamine aqueous solution into the second three-necked flask, then add 99.6g of methyl acetate, start stirring, then add 7.5g of 4-dimethylaminopyridine catalyst, and then dropwise add 196g of 30% NaOH aqueous solution (liquid Alkali), the temperature was kept at 20°C, and the reaction was carried out for 1 h. Add 136g of trans-1,3-dichloropropene into the second three-necked flask, raise the temperature to 60°C, and react for 6h. The molar ratio of the above raw materials n(hydroxylamine):n(methyl acetate):n(4-dimethylaminopyridine):n(NaOH):n(trans-1,3-dichloropropene) is 1:1.1:0.05 :2.1:1.
[0038] Subsequently, 180 g of hydrochloric acid was added, and after stirring, it was refluxed at 60° C. for 2 h. After the s...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap