A-D-A type condensed ring conjugated organic small molecule photosensitizer and application thereof
A photosensitizer and small molecule technology, applied in the field of biomedicine, can solve the problems of high toxicity of cyano groups, etc., and achieve the effects of high-efficiency photothermal therapy, good biological safety and biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Novel A-D-A type fused ring conjugated organic small molecule photosensitizer F8CA among the present invention, its structural formula is as shown in the following formula:
[0023]
[0024] Its synthetic route, as shown in the following formula:
[0025]
[0026] Under the protection of nitrogen, F8-CHO (114mg, 0.10mmol), CA (145mg, 0.60mmol) were dissolved in an appropriate amount of chloroform, and pyridine (0.5mL) was added, and the mixture was reacted at 65°C for 20 hours. After the reaction, the solution was After adding a large amount of methanol, the crude product was obtained by filtration, and the crude product was further purified by column chromatography to obtain the compound F8CA.
[0027] Dissolve the product in CD 2 Cl 2 Medium (about 0.4mL), seal the tube, measure and characterize on Unity Inova-400 NMR instrument: 8.78(m,2H),8.17-8.03(m,4H),7.90-7.82(m,2H),7.77- 7.63(m,4H),7.24-7.12(m,16H),4.47-4.37(m,4H),2.60-2.54(m,8H),1.58(m,12H),1.40(m,6H)...
Embodiment 2
[0029] Preparation and Characterization of Nanomedicines
[0030] DSPE-PEG2000 (6 mg) and photosensitizer F8CA (1.2 mg) were dissolved in 1 mL of THF, and the THF solution was dropped into rapidly stirring 10 mL of deionized water at a rate of 4 mL / h using a syringe pump, and stirred for 2 hours. The mixed solution was dialyzed using a 10,000 molecular weight dialysis bag to obtain a blue clear nanomedicine aqueous solution.
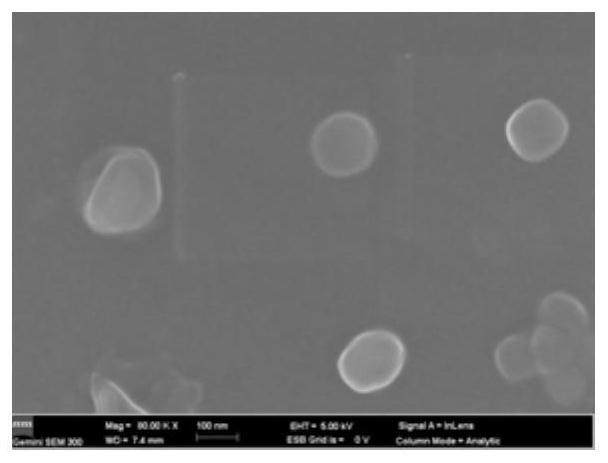
[0031] Characterization of nanomedicine self-assembly morphology by SEM ( figure 1 shown).
[0032] (1) Photothermal heating curve:
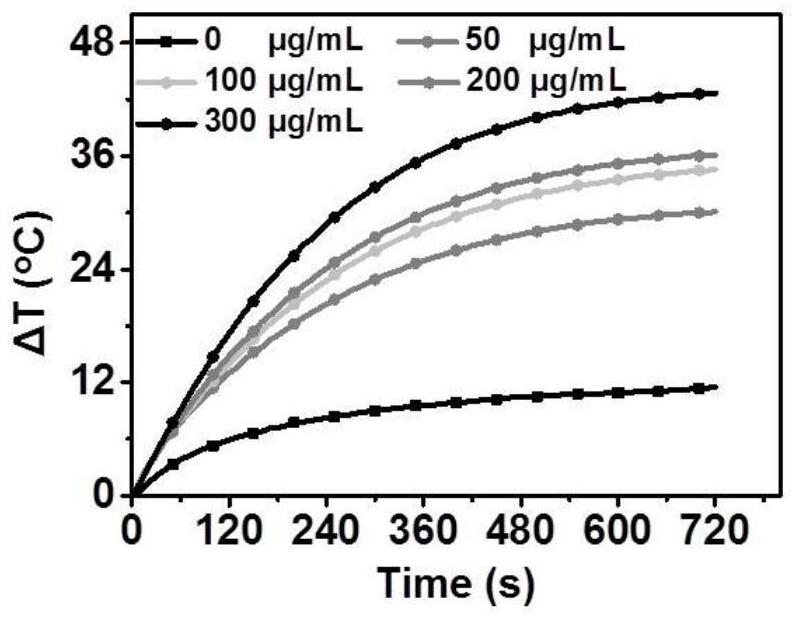
[0033] Using 660nm, 1.0W / cm 2 The laser was irradiated, and the heating curves of five different concentrations of F8CA NPs of 0 μg / mL, 50 μg / mL, 100 μg / mL, 200 μg / mL and 300 μg / mL were drawn.
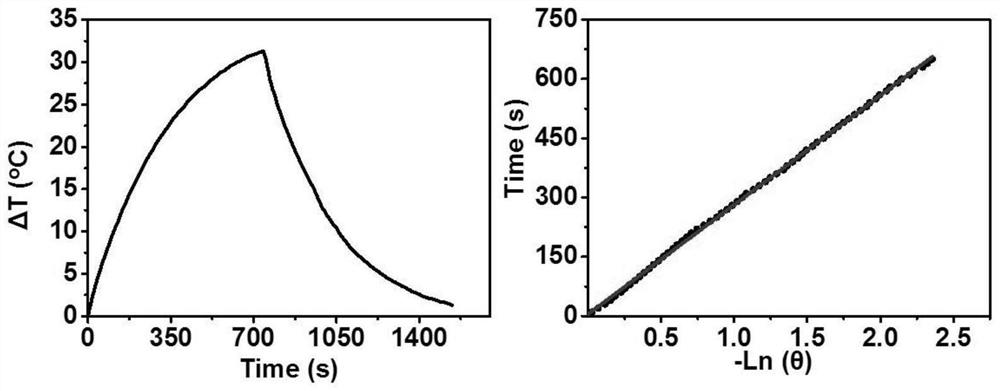
[0034] Such as figure 2 As shown, the heating rate of the F8CA NPs aqueous solution increased with the increase of the concentration, and the temperature increased by more than 40°C at the maximum concentration, which was suf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com