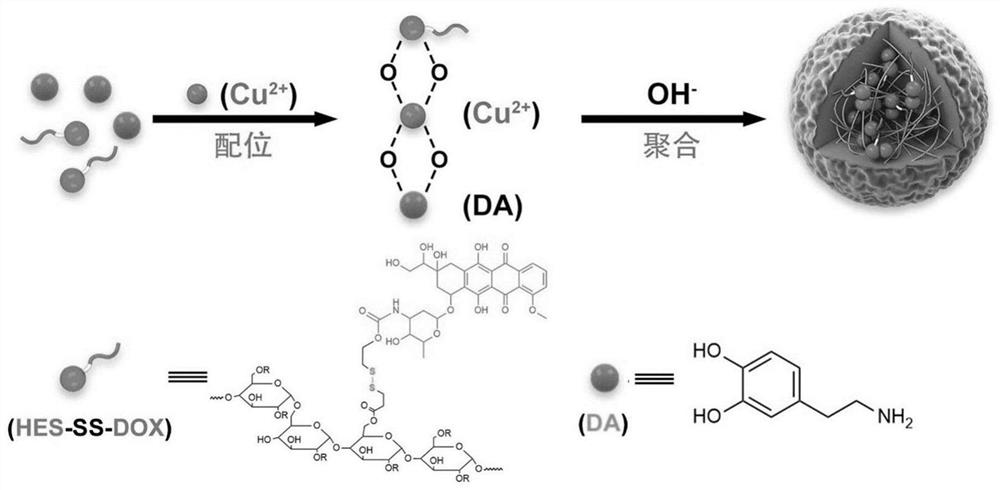
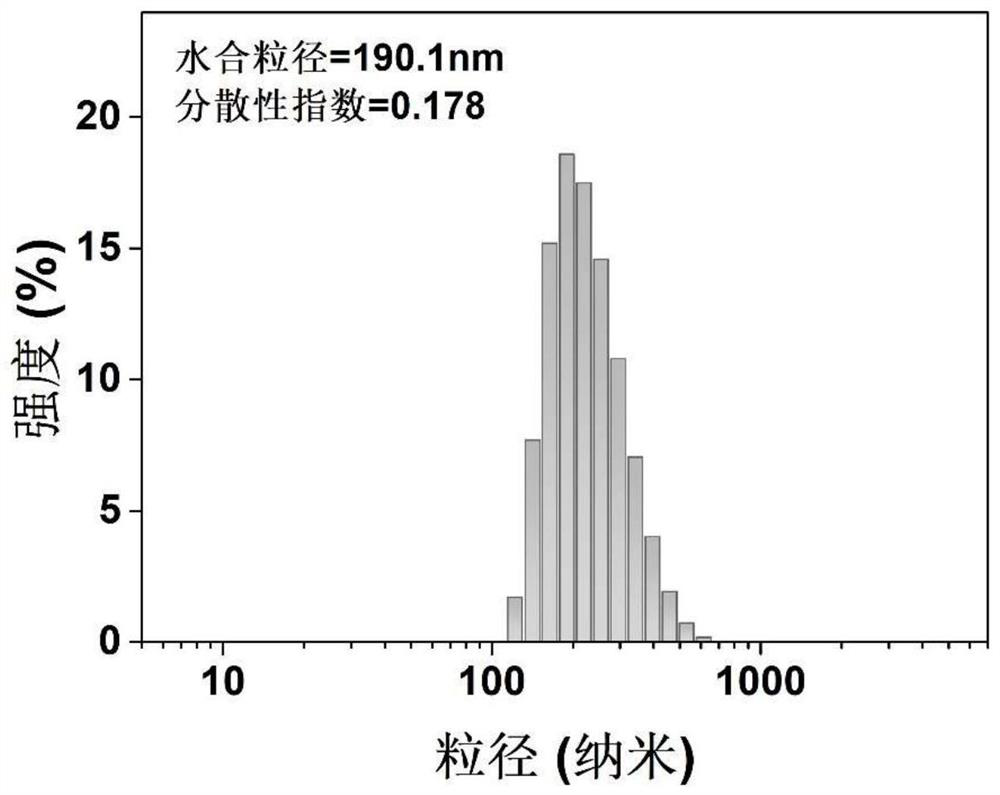
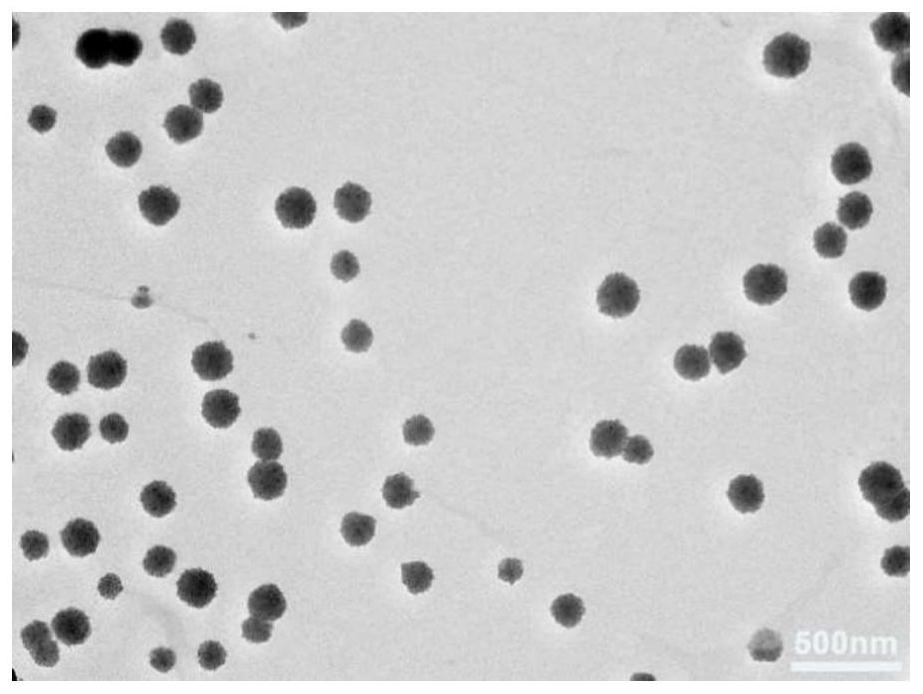
Copper-doped polydopamine nano-drug with stable hydroxyethyl starch prodrug as well as preparation and application of copper-doped polydopamine nano-drug
A technology of polydopamine nanometer and hydroxyethyl starch, which is applied in the direction of nanomedicine, drug combination, nanotechnology, etc., can solve the problems of poor stability of PDA, limited tumor treatment effect, low light and heat absorption, etc., and achieve synergistic chemistry Kinetic therapy, improving PDA stability, enhancing the effect of photothermal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The hydroxyethyl starch prodrug used in the present invention is a reduction-responsive hydroxyethyl starch doxorubicin conjugate linked by a disulfide bond (abbreviated as HES-SS-DOX, further abbreviated as HSD). In some embodiments, the preparation method of HSD comprises the following steps:
[0046] (1) The hydroxyl group on hydroxyethyl starch and the carboxyl group on 3,3'-dithiodipropionic acid (DTDPA) undergo an esterification reaction in anhydrous dimethyl sulfoxide, and react at a temperature of 25-45°C The intermediate product was obtained in 24-72 hours. The molecular weight of the hydroxyethyl starch in the hydroxyethyl starch prodrug is 40-200kDa, preferably 130kDa.
[0047] (2) After the intermediate product in step (1) is separated and purified, the carboxyl group on the intermediate product and the amino group on the doxorubicin undergo further amide reaction in anhydrous dimethyl sulfoxide to obtain the reduction-responsive hydroxyethyl starch The dr...
Embodiment 1
[0062] A hydroxyethyl starch prodrug-stabilized copper-doped polydopamine nanoparticle, its preparation comprising the steps of:
[0063] (1) Weigh 3,3'-dithiodipropionic acid (DTDPA, 2.95g), dicyclohexylcarbodiimide (DCC, 579mg), 4-dimethylaminopyridine (DMAP, 171mg) in 100mL single port In the bottle, 20 mL of anhydrous dimethyl sulfoxide was added thereto, and stirred at room temperature for 2 hours to obtain carboxyl-activated 3,3'-dithiodipropionic acid. Then 5 g of dried hydroxyethyl starch (HES130 / 0.4, indicating that its average molecular weight is 130 kDa, and the molar substitution degree of hydroxyethyl group is 0.4) was weighed into the reaction system, and reacted at room temperature for 48 hours. After the reaction is completed, it is washed three times with a mixed solution of isopropanol / petroleum ether, and vacuum-dried to obtain the HES-DTDPA intermediate.
[0064] (2) Weigh HES-DTDPA (540 mg) and dissolve it in 20 mL of anhydrous dimethyl sulfoxide, add 1-(...
Embodiment 2
[0070]A hydroxyethyl starch prodrug-stabilized copper-doped polydopamine nanoparticle, its preparation comprising the steps of:
[0071] (1) Weigh 3,3'-dithiodipropionic acid (DTDPA, 295mg), dicyclohexylcarbodiimide (DCC, 57.9mg), 4-dimethylaminopyridine (DMAP, 17.1mg) in 25mL 5 mL of anhydrous dimethyl sulfoxide was added to the single-necked bottle, and stirred at room temperature for 2 hours to obtain carboxyl-activated 3,3'-dithiodipropionic acid. Then weighed dry hydroxyethyl starch 0.5 (HES40 / 0.5, indicating that its average molecular weight is 40kDa, and the molar substitution degree of hydroxyethyl is 0.5) was added into the reaction system, and reacted at room temperature for 72h. After the reaction is completed, it is washed three times with a mixed solution of isopropanol / petroleum ether, and vacuum-dried to obtain the HES-DTDPA intermediate.
[0072] (2) Weigh HES-DTDPA (108 mg) and dissolve it in 10 mL of anhydrous dimethyl sulfoxide, add 1-(3-dimethylaminopropyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com