Application of 4-methylumbelliferone in preparation of anti-allergic drugs
A methylumbelliferone and anti-allergic technology, which is applied in the field of biopharmaceuticals, can solve the problems of large side effects and poor anti-allergic drug effects, and achieve the effect of reducing degranulation and alleviating the symptoms of local and systemic allergic reactions in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
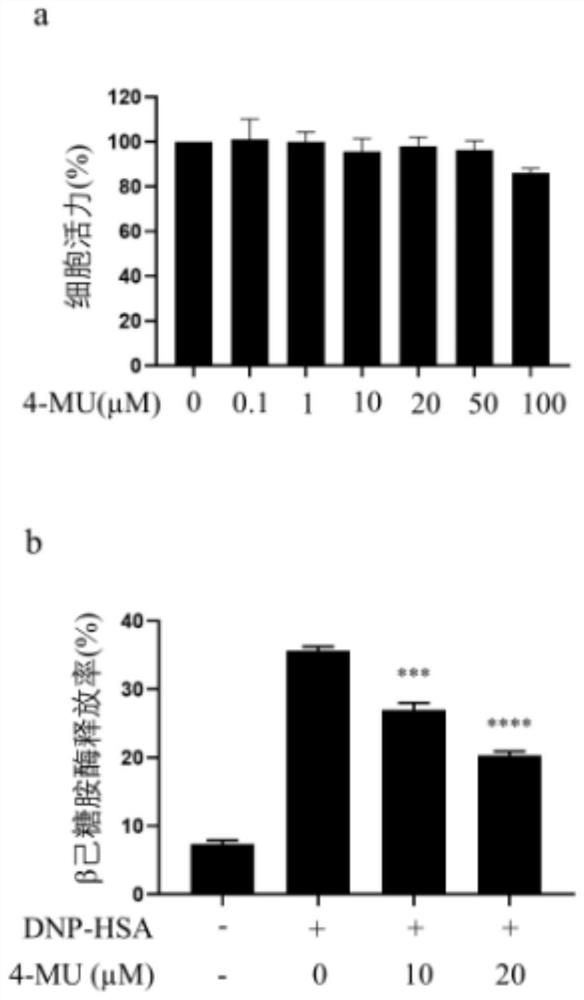
[0020] Inhibitory effect of 4-MU on rat basophil (RBL-2H3) degranulation:
[0021] Mouse basophils are widely used in the diagnosis and immunotherapy of allergies. RBL-2H3 is easy to culture and is widely used in degranulation and signaling pathways, mast cell stabilizers, physicochemical properties of FcεR and its cytoskeleton Interaction studies etc. RBL-2H3 has high-affinity IgE receptors, which can be activated to secrete histamine and other transmitters by accumulating these receptors or synergizing with calcium ionophores.
[0022] In order to verify the inhibitory effect of 4-MU on the degranulation of rat basophils (RBL-2H3), its specific implementation is as follows:
[0023] Cell culture: Rat basophilic leukemia-2h3 cell lines (RBLs) were purchased from Cellcook Biotechnology Company (Guangzhou, China) and cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 4.0 mM l-glutamine Amide, 1.0mM sodium pyruvate, 100U / ml penicillin, 100μg / ml streptomycin, non-es...
Embodiment 2
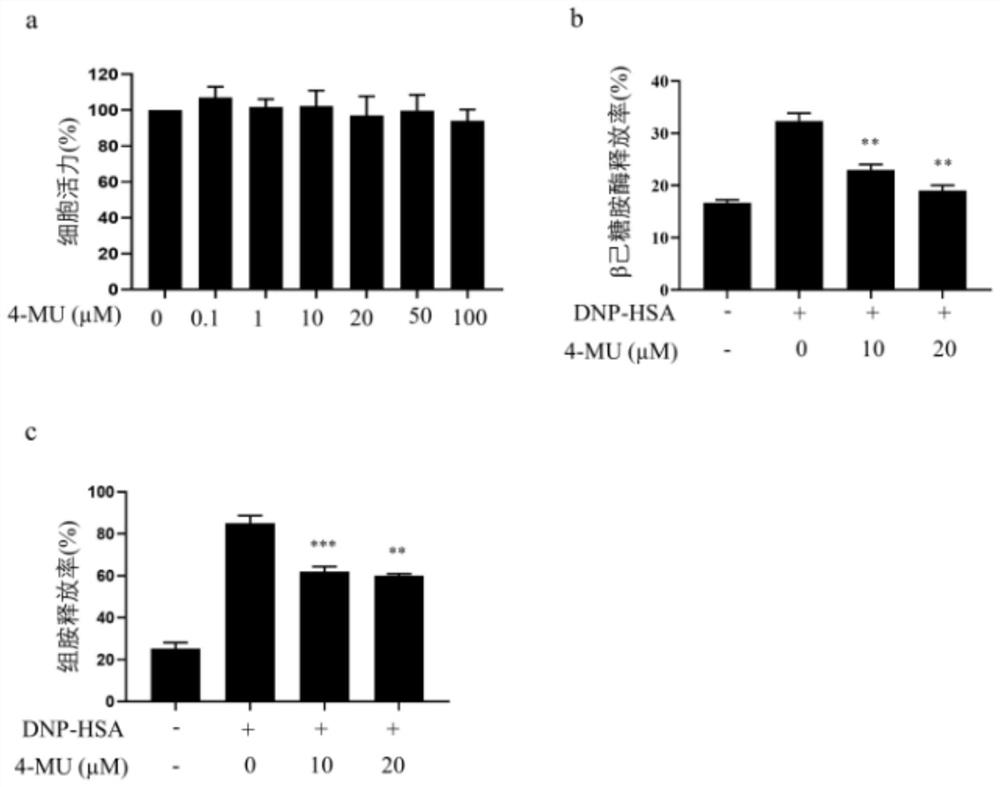
[0028] 4-MU inhibits degranulation of bone marrow-derived mast cells (BMMCs):
[0029] Cell culture: BMMCs were isolated from Balb / c mice (purchased from Guangdong Medical Experimental Animal Center), cultured in RPMI (Hyclone), 10% FBS (Gibico), 1% double antibody, 10ng / ml IL-3 and SCF4 -6 weeks.
[0030] CCK8 detects the toxicity of cells to 4-MU: BMMC (1×104 / well) were seeded in a 96-well plate, T-5224 was added after 24 hours, and incubation was continued for 24 hours. Then add 10ul CCK8 reagent, incubate for 1 hour, and finally measure the absorbance (OD value) with a microplate reader at a wavelength of 450nm.
[0031] Release of β-aminoglycosidase: Inoculate BMMC in a 24-well plate, incubate overnight with anti-DNP IgE for sensitization, incubate with 4-MU for 1 hour, stimulate with DNP-HSA (sigma) for 30 minutes, take 50ul supernatant and 50ul The substrate was reacted at 37°C for 90mins. The absorbance was measured at 405nm. After the supernatant was discarded, 500...
Embodiment 3
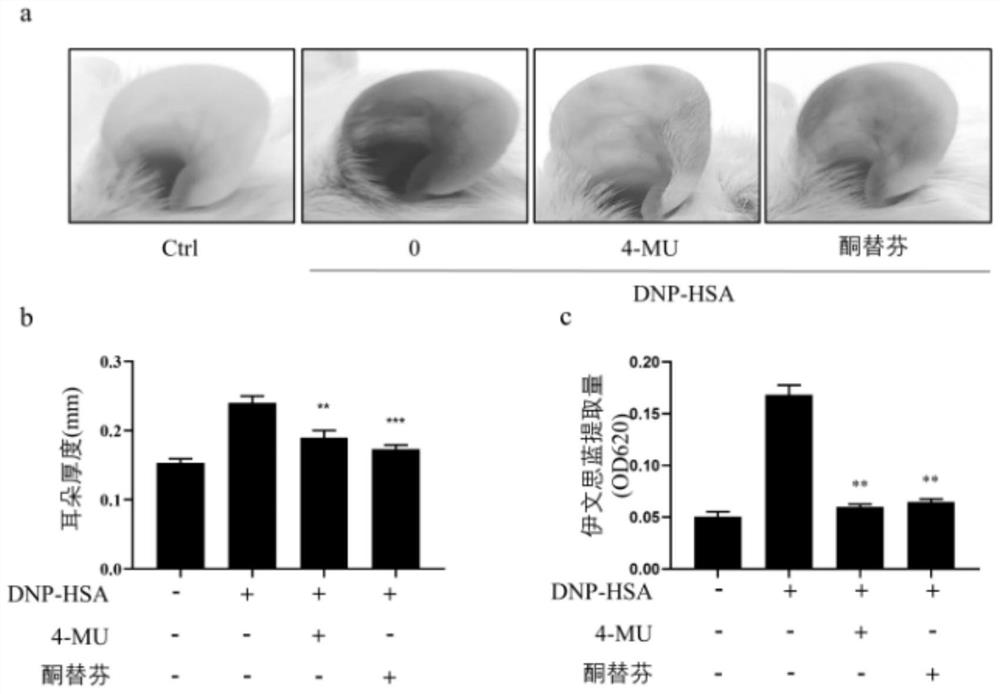
[0035] Effects of 4-MU on passive skin hypersensitivity in mice:
[0036] Breeding of BALB / c mice: Female mice (4-5 weeks old BALB / C) were purchased from the Guangdong Medical Laboratory Animal Center (Foshan, Guangdong) and placed in a temperature (24±1°C) and humidity (55±10 %) in a relatively stable SPF environment for 1 week. Mice were used to isolate bone marrow-derived mast cells (BMMC) and to perform a passive cutaneous anaphylaxis (PCA) model and an active systemic anaphylaxis (ASA) model.
[0037] Mice were injected with anti-DNP-IgE 500ng / ear. 24 hours later, 4-MU (50 mg / kg) or ketotifen (50 mg / kg, positive control group) was intraperitoneally injected (i.p.) into the ear (N=5 / group). One hour later, the mice were injected with 200 μg of DNP-HSA PBS containing 0.5% Evans blue dye into the tail vein. Mice were euthanized 1 h after DNP-HSA injection. Ears were taken and placed in 700 μl formamide for extraction at 65°C for 12 hours. Dye absorbance was measured at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com