Preparation method of amino hydroxyurea
A technology of semicarbazide and urethane, which is applied in the field of preparation of semicarbazide, can solve the problems of no practical value in large-scale production, low yield, and high cost, and achieve low production cost, high reaction yield, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
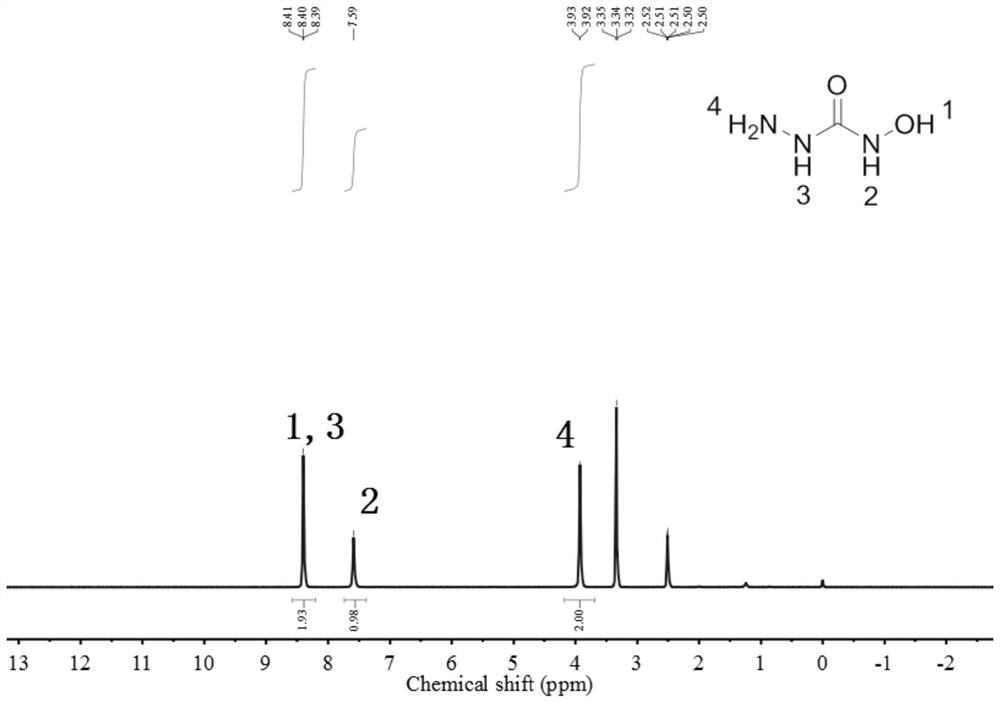
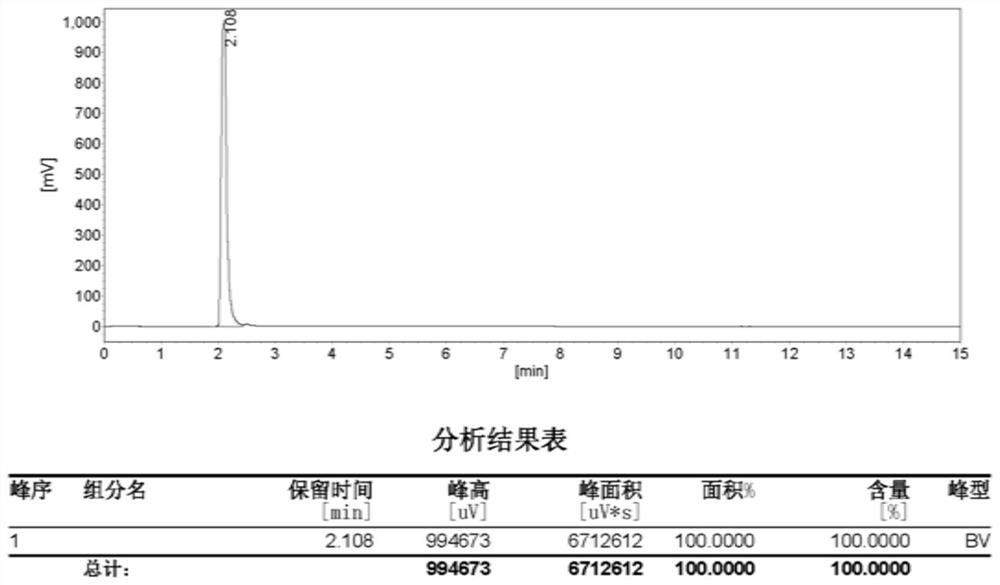
[0025] refer to Figure 1 to Figure 3 , the present disclosure provides a preparation method of hydroxysemicarbazide, the preparation method may include: under urethane exchange conditions and in the presence of an acid-binding agent, contacting a substance of formula A and a substance of formula B in a solvent to obtain materials after contact.
[0026]
[0027] In the present invention, in the substance of formula A, R is any one of the following substituents:
[0028]
[0029] The substance of formula B is at least one of hydroxylamine, hydroxylamine hydrochloride, hydroxylamine sulfate, hydroxylamine phosphate, hydroxylamine sulfonate and hydroxylamine nitrate. The substance of formula A is preferably at least one of the The substance of formula B is preferably at least one of hydroxylamine, hydroxylamine hydrochloride and hydroxylamine sulfate.
[0030] Optionally, the molar ratio of the substance of formula A to the substance of formula B is 1:1-3.
[0031] A...
Embodiment 1
[0044] Under urethane exchange conditions and in the presence of an acid-binding agent (sodium hydroxide), a substance of formula A (ethyl carbazate) and a substance of formula B (hydroxylamine) are contacted in a solvent (ethanol) to obtain contact After the material; the molar ratio of the substance of the formula A and the substance of the formula B is 1:2.4; the urethane exchange conditions include: the temperature is 10 ° C, the time is 24h; relative to each mole of the formula For the substance of A, the amount of the solvent used is 0.79 L; the amount of the acid-binding agent used is 1.2 mol.
[0045] The contacted materials were subjected to reduced-pressure rotary evaporation and first drying to obtain a crude product; the temperature of the reduced-pressure rotary evaporation was 60° C.; the temperature of the first drying was 80° C. for 16 hours.
[0046] Mix and re-dissolve the crude product with ethanol and water, then carry out recrystallization, filtration and ...
Embodiment 2
[0048] Under urethane exchange conditions and in the presence of an acid-binding agent (sodium carbonate), a substance of formula A (methyl carbazate) and a substance of formula B (hydroxylamine hydrochloride) are contacted in a solvent (ethanol) to obtain contact After the material; the molar ratio of the substance of the formula A and the substance of the formula B is 1:1.2; the urethane exchange conditions include: room temperature, the time is 48h; relative to the substance of the formula A per mole , the amount of the solvent is 0.833L; the amount of the acid-binding agent is 1.198mol.
[0049] The contacted materials were subjected to reduced-pressure rotary evaporation and first drying to obtain a crude product; the temperature of the reduced-pressure rotary evaporation was 65° C.; the temperature of the first drying was 80° C. for 16 hours.
[0050] Mix and re-dissolve the crude product with ethanol and water, then carry out recrystallization, filtration and second dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com