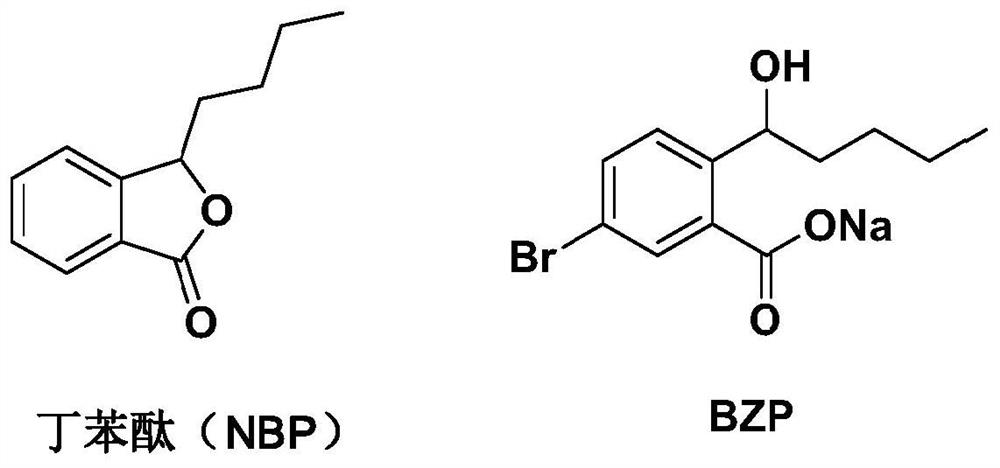
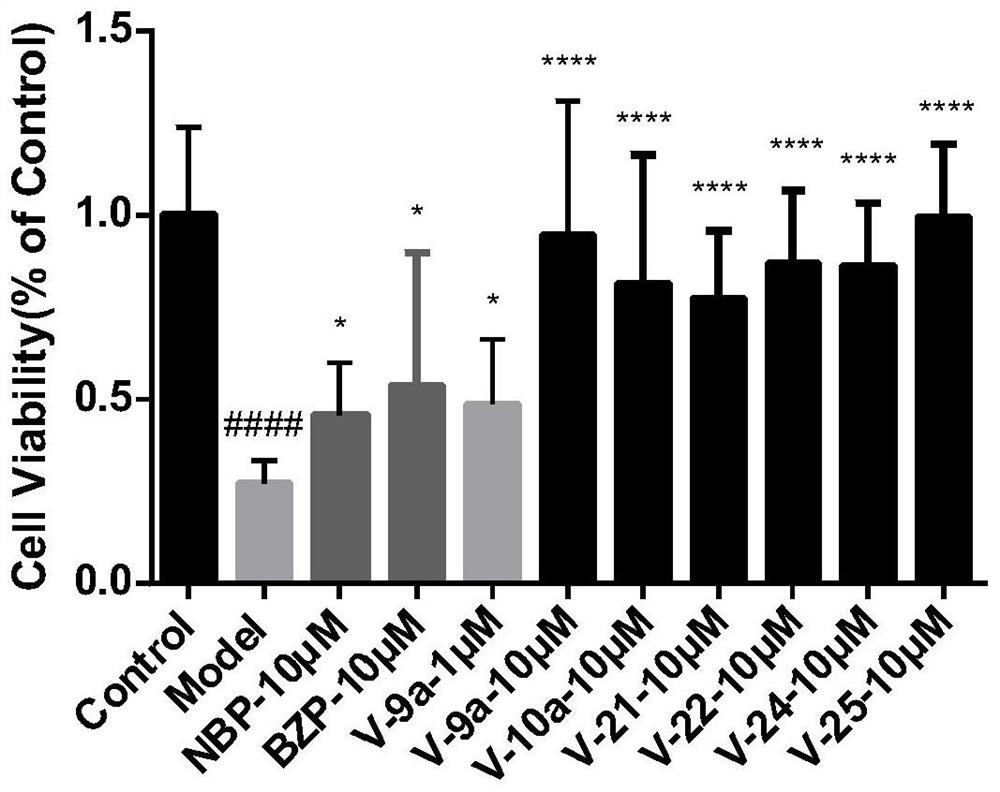
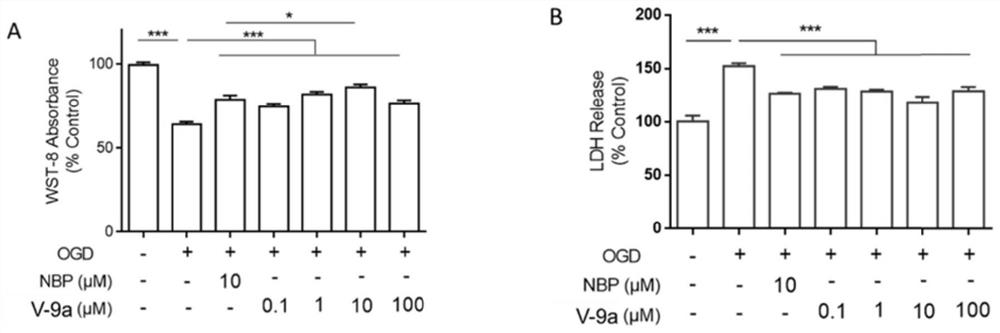
Tetrazole derivative, preparation, pharmaceutical composition containing tetrazole derivative and application of tetrazole derivative
A technology of tetrazole derivatives, which is applied in the field of preparation of tetrazole derivatives, can solve problems such as lack of treatment strategies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Example 1. Synthesis of Intermediates 1-3
[0153]
[0154] Add raw material 1-1 (13.2g, 0.1mol), NBS (N-bromosuccinimide) (44.5g, 0.25mol), AIBN (azobisisobutyronitrile) (720mg, 3mmol), carbon tetrachloride (20ml), heated to reflux for 16h, cooled to room temperature, and concentrated under reduced pressure to remove carbon tetrachloride to obtain a mixture containing intermediate 1-2. Add water and ethanol (100ml: 100ml) to the mixture, react at 70 degrees Celsius for 4h, extract with ethyl acetate to obtain an organic phase, dry over anhydrous sodium sulfate, concentrate under reduced pressure and evaporate to dryness ethyl acetate, the obtained crude product is subjected to silica gel column chromatography (acetic acid Ethyl ester: petroleum ether = 1:50-1:10) was purified to obtain about 8.4 g of white to pale yellow crystalline intermediate 1-3, with a yield of 40% and a purity of more than 99%. HRMS:m / z(ESI)calcd for C 8 h 6 N 2 O[M+H] + 147.05found: 147....
Embodiment 2
[0155] Embodiment 2: the synthesis of intermediate 1-5
[0156]
[0157] Step 1: add intermediate 1-3 (2.8g, 20mmol), ethylene glycol (6.2g, 0.1mol), toluene (20ml), p-toluenesulfonic acid (275mg, 1.6mmol) to reflux in a single-necked bottle, Use a water separator to remove water until the water volume in the water separator no longer increases, and cool to room temperature. Saturated sodium bicarbonate solution was added until the water layer was neutral or alkaline, the water layer was separated, and the organic layer was concentrated under reduced pressure to obtain 4.5 g of light yellow oily semi-solid intermediate 1-4. Yield 89%. The purity is greater than 99%.
[0158] Step 2: Add intermediate 1-4 (0.7g, 3.93mmol), sodium azide (1.28g, 19.65mmol), ammonium chloride (1.05g, 19.65mmol), and DMF (10ml) into a single-necked flask, Heat to reflux for 12 hours, cool to room temperature, add 1N hydrochloric acid to quench, stir at room temperature for 1 hour, TLC confirms...
Embodiment 3
[0159] Embodiment 3: the synthesis of intermediate 2-3
[0160]
[0161] With reference to the steps of Example 1, 5-(trifluoromethyl)-2-methylbenzonitrile 2-1 (18.5 g, 0.1 mol) was used as raw material to obtain 15.2 g of solid 2-3 with a yield of 76% and a purity greater than 99%, HRMS: m / z (ESI) calcd for C 9 h 4 f 3 NO[M+H] + 200.02found: 200.25.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com