Novel crystal form of 2-aminopyridine derivative and preparation method thereof
A crystallization, D-type technology, used in organic chemical methods, medical preparations containing active ingredients, separation/purification of carboxylic acid compounds, etc., can solve problems such as low positive rate, and achieve simple preparation methods and good thermal stability. , the effect of good liquidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0104] The preparation of embodiment 1 formula Ia compound
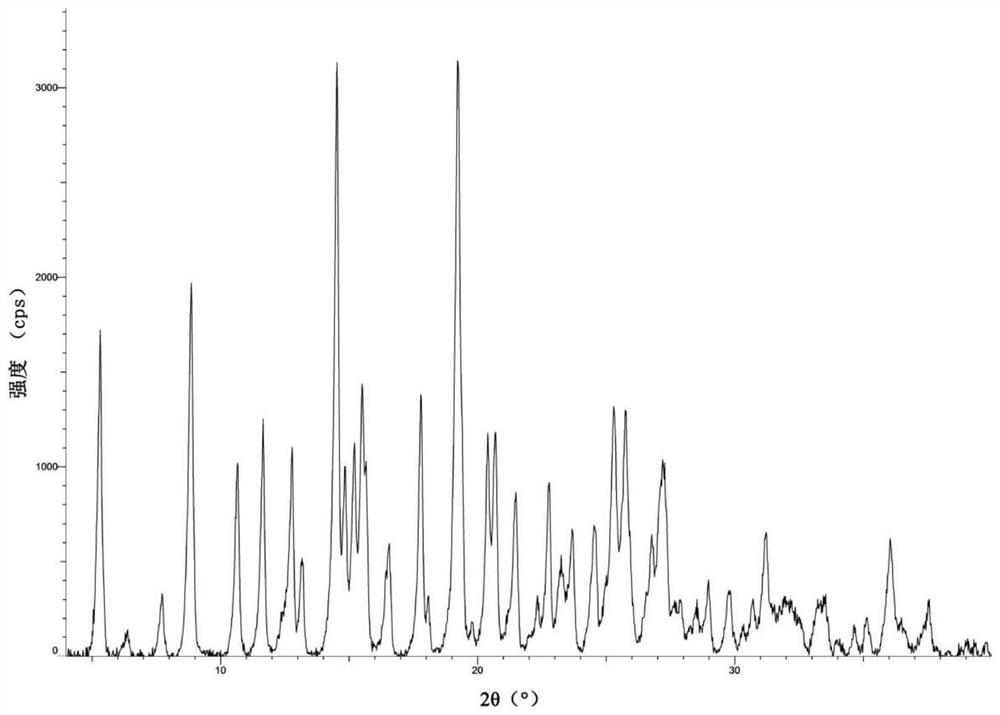
[0105] Dissolve 0.01mol of the compound of formula I in 90mL of methanol, add 10mL of citric acid-methanol solution with a concentration of 1.1mol / L under reflux and stir, and react for 1 hour, the reaction solution precipitates a solid, and continues to reflux hours, filtered, and the separated solid stirred at reflux in ethanol hour, filtered, and the separated solid was dried with air blast at 85°C under normal pressure to obtain crystals of the compound of formula Ia. The X-ray powder diffraction pattern using CuKa radiation was shown as type A crystals of WO2017016514, such as Figure 11 Shown, the diffraction peak in its X-ray diffraction pattern has following characteristics:
[0106]
[0107]
[0108] 1 H-NMR (500MHz, DMSO-d 6 ): δ=7.83(s, 1H), 7.57(t, J=4.95Hz, 1H), 7.55(d, J=1.45Hz, 1H), 7.46(t, J=8.7Hz, 1H), 6.81(d ,J=1.2Hz,1H),6.38(s,1H),5.98(q,J=6.7Hz,1H),5.73(brs,2H),4.79(brs,1H),4.26(d,J=13...
Embodiment 2
[0109] The preparation of the H type crystal of embodiment 2 formula Ia compound
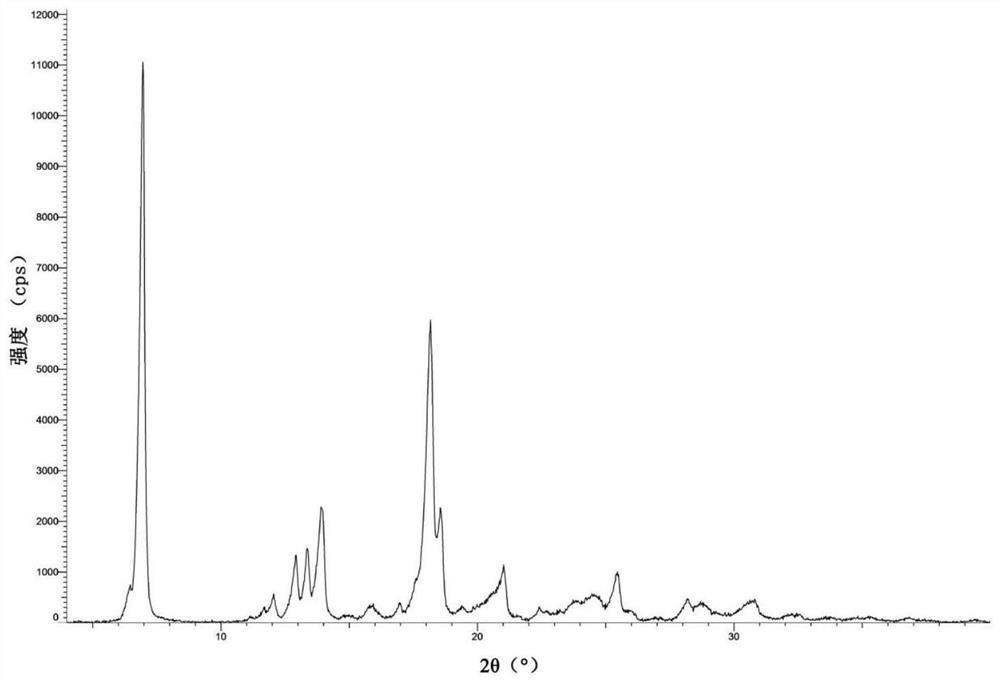
[0110] Weigh 250 mg of the compound of formula Ia obtained in Example 1 into a 50 mL one-necked flask, add 40 mL of acetonitrile-water mixed solvent (acetonitrile: water (v / v) = 0.25:1) to obtain a suspension, then stir at room temperature for 24 hours, filter , and the separated solid was air-dried at 50° C. for 2 hours to obtain type H crystals of the compound of formula Ia.
[0111] 1 H-NMR (500MHz, DMSO-d 6 ):δ=7.81(s,1H),7.58(dd,J=9.0,4.9Hz,1H),7.53(d,J=1.8Hz,1H),7.47(t,J=8.7Hz,1H),6.78 (d,J=1.9Hz,1H),6.37(s,1H),5.97(q,J=6.7Hz,1H),5.75(s,2H),4.78(d,J=6.3Hz,1H),4.30 –4.19(m,1H),3.69(s,3H),3.30(d,J=12.2Hz,1H),3.23(d,J=12.6Hz,1H),3.18–3.08(m,2H),2.95( td,J=12.4,3.8Hz,1H),2.58(d,J=15.1Hz,2H),2.51(d,J=15.1Hz,2H),1.77(d,J=6.6Hz,3H),1.20( d, J=6.9Hz, 3H).
Embodiment 3
[0112] The preparation of the H type crystal of embodiment 3 formula Ia compound
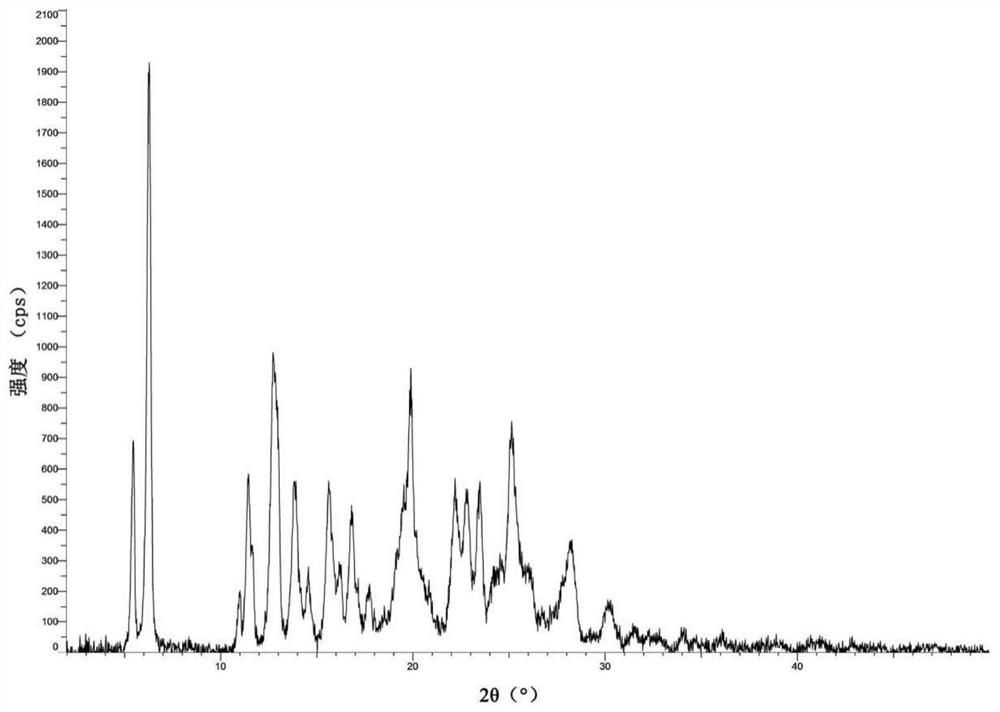
[0113] Weigh 250 mg of the compound of formula Ia obtained in Example 1 into a 50 mL one-necked flask, add 40 mL of acetonitrile-water mixed solvent (acetonitrile: water (v / v) = 9:1) to obtain a suspension, then stir at room temperature for 24 hours, filter , and the separated solid was air-dried at 50° C. for 2 hours to obtain type H crystals of the compound of formula Ia.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com