Method for synthesizing 3-hydroxy-3-allyl oxoindole and 3-hydroxy-3-allene oxoindole
An indole oxide, allyl technology, applied in chemical instruments and methods, organic chemistry methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve problems such as difficulties and limited expansion of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0094] In the present invention, the preparation method of the chiral zinc complex preferably comprises the following steps:
[0095] a) After reacting the zinc salt, the basic compound and the ligand in a solvent, a chiral zinc complex is obtained.
[0096] In the present invention, the zinc salt preferably includes one or more of zinc bromide, zinc fluoride, zinc chloride, zinc trifluoromethanesulfonate, zinc nitrate, zinc sulfate and zinc acetate, more preferably bromine Zinc chloride, zinc fluoride, zinc chloride, zinc trifluoromethanesulfonate, zinc nitrate, zinc sulfate, or zinc acetate.
[0097] In the present invention, the basic compound preferably includes cesium carbonate, lithium carbonate, sodium carbonate, potassium carbonate, potassium tert-butoxide, triethylenediamine, triethylamine, piperidine, 1,8-diazabicyclo [5.4.0] One or more of undec-7-ene, N,N-diisopropylethylamine and N-ethylmorpholine, more preferably cesium carbonate, lithium carbonate, sodium carbo...
Embodiment 1
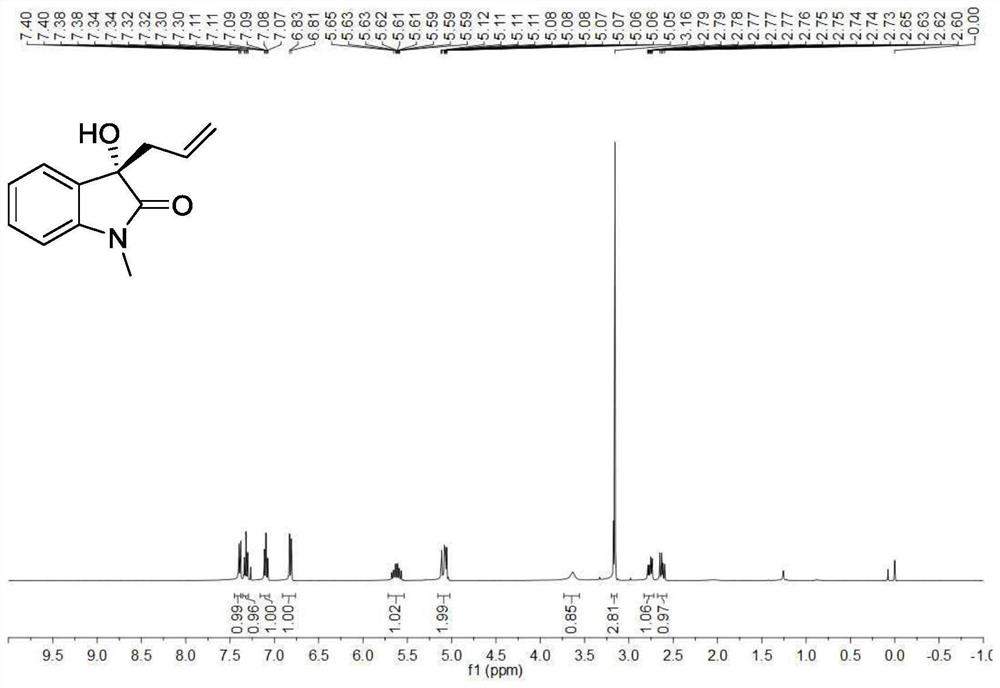
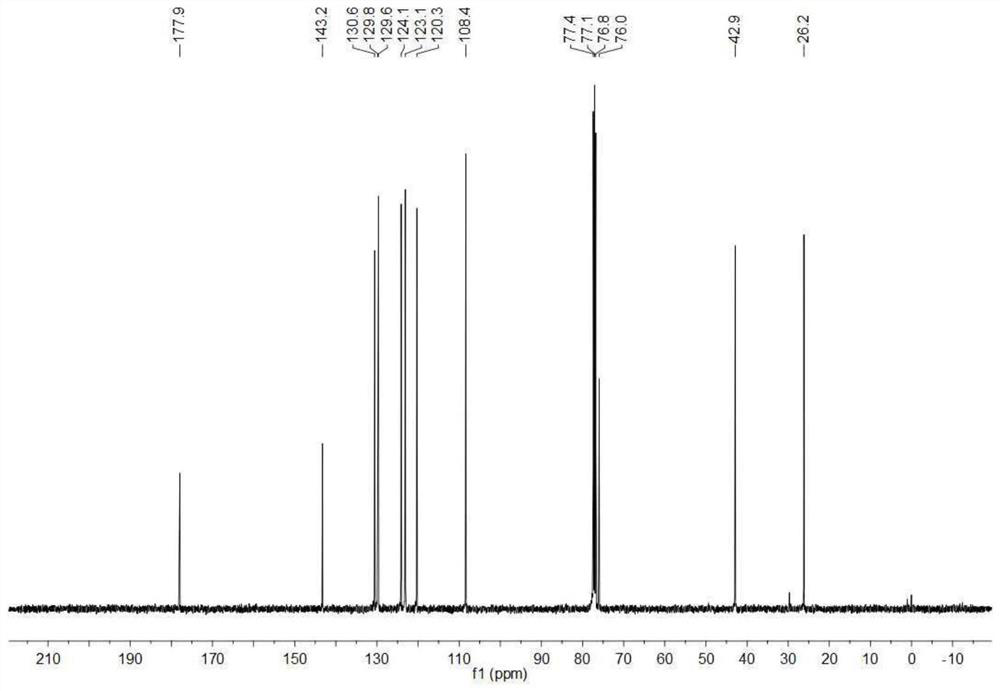
[0191] In a 10mL reaction tube, add zinc triflate (3.6mg, 0.01mmol), ligand (L 2 , 4.6mg, 0.01mmol), acetone (1.0mL), cesium carbonate (3.3mg, 0.01mmol) were stirred at room temperature for 2h. Then, isatin 1a (16.1mg, 0.1mmol), allylboronic acid pinacol ester 2a (42.0mg, 0.25mmol) and 10 microliters of methanol were added successively at 10°C. After the reaction was completed (TLC tracking detection), Extracted with ethyl acetate, extracted with saturated brine, dried over anhydrous sodium sulfate, and spin-dried the obtained residue to pass through the column with petroleum ether / ethyl acetate system as eluent to obtain a white solid (S)-3a (98% yield , 19.9 mg, 93% ee).
[0192] Utilize nuclear magnetic resonance to analyze the target product (S)-3a that obtains in embodiment 1, obtain its proton nuclear magnetic resonance spectrum figure, as figure 1 shown.
[0193] see figure 1 , figure 1 It is the proton nuclear magnetic resonance spectrum of the target product (S)-...
Embodiment 2
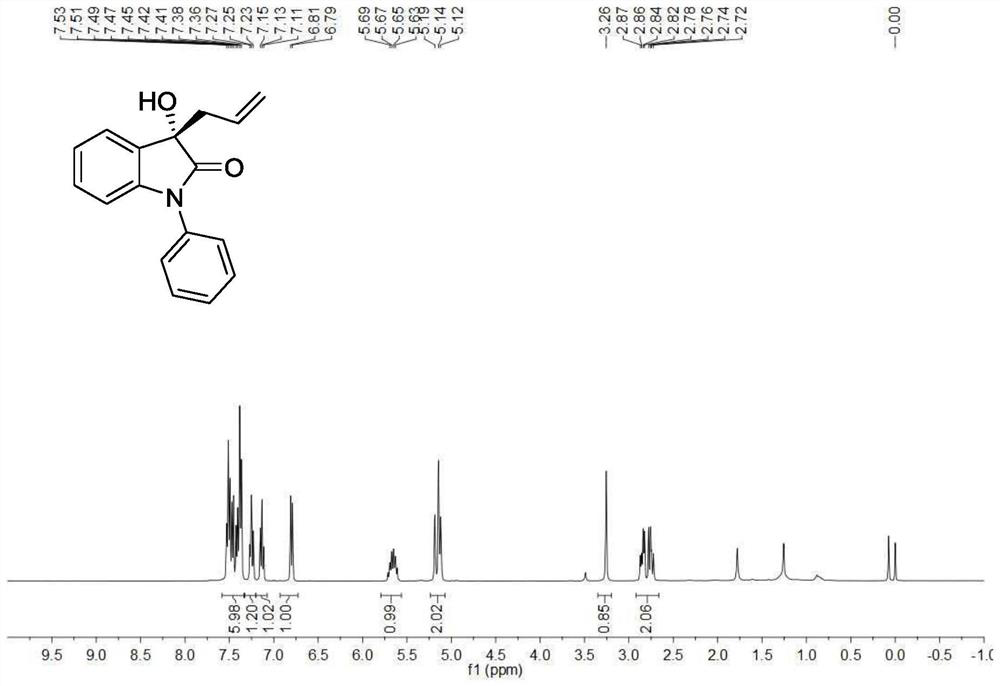
[0199] In a 10mL reaction tube, add zinc triflate (3.6mg, 0.01mmol), ligand (L 2 , 4.6mg, 0.01mmol), acetone (1.0mL), cesium carbonate (3.3mg, 0.01mmol) were stirred at room temperature for 2h. Then, isatin 1b (22.3mg, 0.1mmol), allylboronic acid pinacol ester 2a (42.0mg, 0.25mmol) and 10 microliters of methanol were added successively at 10°C. After the reaction was completed (TLC tracking detection), Extracted with ethyl acetate, extracted with saturated brine, dried over anhydrous sodium sulfate, and spin-dried the obtained residue to pass through the column with petroleum ether / ethyl acetate system as eluent to obtain a white solid (S)-3b (97% yield , 25.7 mg, 89% ee).
[0200] Utilize nuclear magnetic resonance to analyze the target product (S)-3b that obtains in embodiment 2, obtain its proton nuclear magnetic resonance spectrum figure, as image 3 shown.
[0201] see image 3 , image 3 The proton nuclear magnetic resonance spectrum of the target product (S)-3b pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com