Preparation method of dapagliflozin impurity
A technology of impurities and compounds, applied in the field of drug synthesis, can solve problems such as unfavorable reactions, easy precipitation of materials, and contamination, and achieve the effects of low preparation cost, stable process, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
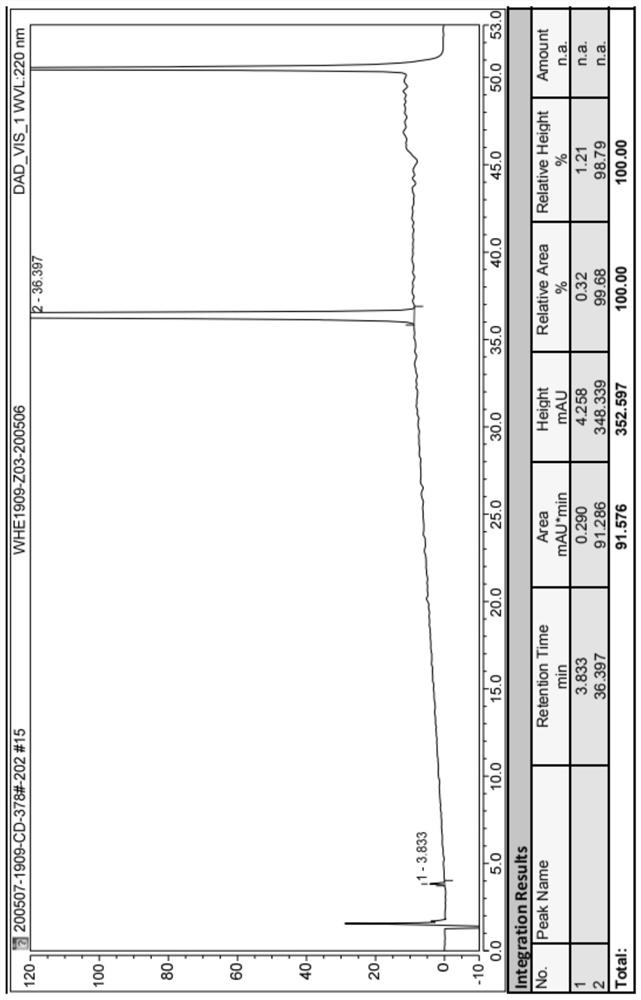
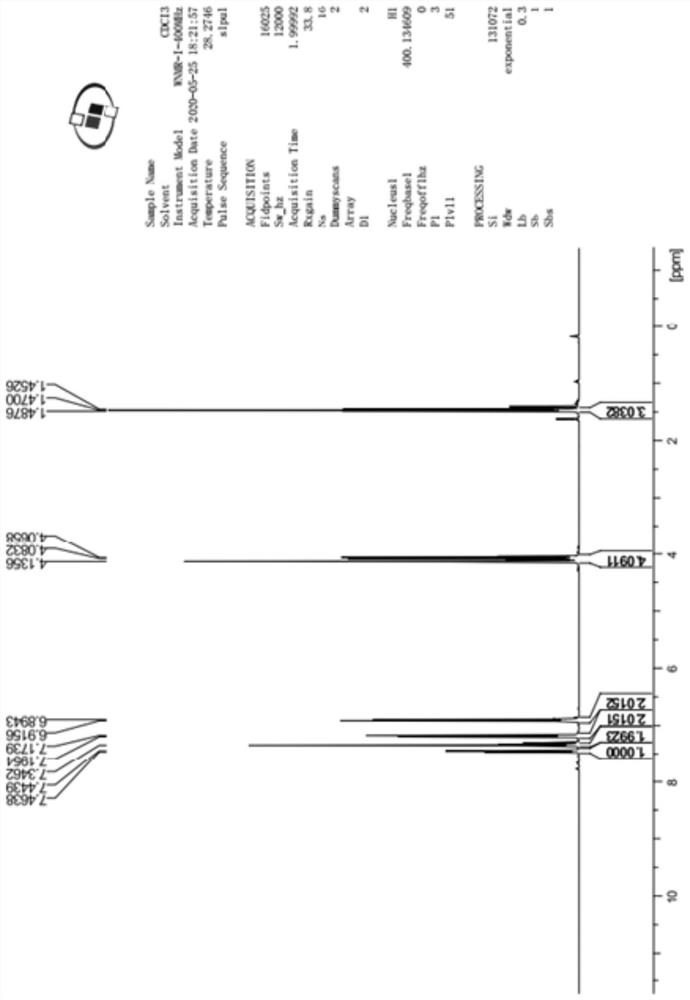
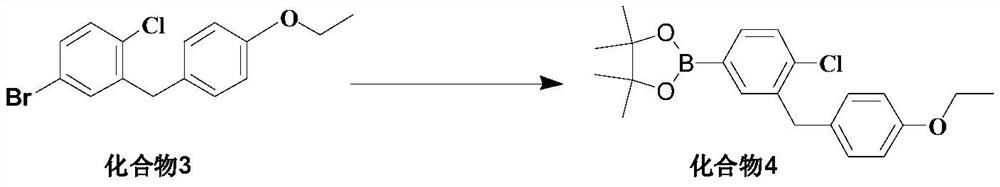
[0034] To a dry 250mL three-necked flask, add 80mL of tetrahydrofuran (8mL / g), start stirring, and then add 5-bromo-2-chloro-4'-ethoxydiphenylmethane (Compound 3) (10.0g, 30.7mmol, 1.0eq). After nitrogen displacement protection, the temperature was lowered to -80~-70°C. After the internal temperature of the system drops to -80~-70°C, add (2.5mol / L) n-BuLi solution 13.5mL (1.1eq) dropwise to react, and the temperature is controlled between -80~-70°C during the dropping process. After the dropwise addition was completed, the reaction was continued within the temperature range for 1.5 h to obtain solution A.
[0035] Add methyl pinacol borate (11.7g, 46.1mmol, 1.5eq) into another 250mL three-necked flask and dissolve it in 50mL of tetrahydrofuran to prepare solution B, start stirring, and add the above-prepared solution A to solution B dropwise , During the dropping process, the temperature is controlled between -80 and -70°C, and the reaction is continued within this temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com