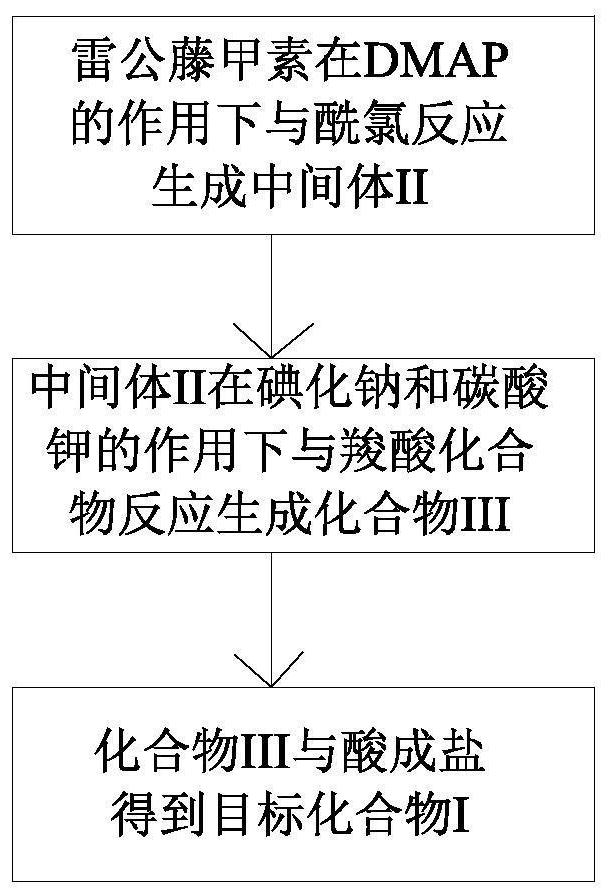
Triptolide prodrug as well as preparation method and medical application thereof
A technology of triptolide and prodrugs, applied in the field of medicinal chemistry, can solve the problems of low content of alkaline phosphatase, unfavorable large-scale preparation, harsh reaction conditions, etc., and achieve good pharmacokinetic properties and good industrial performance. Optimize the foreground and synthesize simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of compound TP-P1
[0046]
[0047] Synthesis of Intermediate II-1: Dissolve triptolide TP (1.8g, 5.0mmol) in 50mL of anhydrous dichloromethane, add DMAP (3.05g, 25.0mmol) at 0°C, and then add chlorine dropwise Acetyl chloride (4.0mL, 50.00mmol), after the dropwise addition, was reacted at 25°C for 12 hours; after the reaction was completed, the reaction solution was washed with 5% dilute hydrochloric acid, saturated sodium bicarbonate and saturated sodium chloride solution successively, and then Dry with anhydrous sodium sulfate; filter with suction, concentrate the filtrate, and purify by column chromatography (dichloromethane:methanol=200:1~100:1) to obtain 1.77g of white solid, with a yield of 88.8%. 1 H NMR(500MHz,MeOD)δ(ppm): 5.14(1H,s),4.78-4.85(2H,m),4.31(2H,d,J=1.0Hz),3.99(1H,d,J=3.2Hz ),3.67(1H,d,J=2.6Hz),3.53(1H,d,J=5.7Hz),2.79-2.81(1H,m),2.24-2.32(2H,m),2.07-2.12(1H, m),1.88-1.98(2H,m),1.50-1.54(1H,m),1.31-1.39(1H,m),1.05(3H,s),0.98(3H,d,J=7.0...
Embodiment 2
[0053] Synthesis of compound TP-P2
[0054]
[0055] Synthesis of Intermediate III-2: Compound II-1 (100mg, 0.23mmol) was dissolved in 10mL of anhydrous DMF, sodium iodide (86mg, 0.46mmol) and 4-methyl-1-piperazineacetic acid (73mg , 0.46mmol); after reacting at 25°C for 40 minutes, potassium carbonate (32mg, 0.23mmol) was added, and then heated to 50°C for 4 hours; after the reaction was completed, the reaction solution was poured into water, extracted with ethyl acetate, and organic layer, washed successively with aqueous sodium bicarbonate solution and saturated sodium chloride solution, and dried over anhydrous sodium sulfate; filtered with suction, concentrated the filtrate, and purified by column chromatography (dichloromethane:methanol=100:1~40:1) to obtain a white solid 93mg, yield 72.3%; 1 H NMR (500MHz, MeOD-d 6 )δ(ppm):5.12(1H,s),4.81-4.85(2H,m), 4.77-4.80(2H,m),3.98(1H,d,J=3.1Hz),3.65(1H,d,J =2.8Hz),3.50(1H,d,J=5.7Hz), 3.47(2H,s),2.79-2.83(1H,m),2.66-2.78(8H,...
Embodiment 3
[0059] Synthesis of Compound TP-P3
[0060]
[0061] Synthesis of compound TP-P3: Compound II-1 (100 mg, 0.23 mmol) was dissolved in 10 mL of anhydrous DMF, and sodium iodide (86 mg, 0.46 mmol) and 2-piperidinylacetic acid (66 mg, 0.46 mmol) were added; After reacting at 25°C for 40 minutes, potassium carbonate (32mg, 0.23mmol) was added, and then heated to 50°C for 4 hours; Wash with aqueous sodium hydrogen solution and saturated sodium chloride solution, dry over anhydrous sodium sulfate; filter with suction, concentrate the filtrate, and purify by column chromatography (dichloromethane:methanol=100:1~40:1) to obtain 67 mg of white solid, yield 53.4 %; 1 H NMR (500MHz, CDCl 3 )δ (ppm): 5.11 (1H, s), 4.81-4.85 (2H, m), 4.65-4.73 (2H, m), 3.84 (1H, d, J = 3.1Hz), 3.64 (2H, d, J =4.2Hz), 3.56(1H,d,J=2.8Hz),3.48(1H,d,J=5.7Hz),2.87-2.98(4H,m),2.32-2.36(1H,m),2.16-2.26 (2H,m),1.89-1.95(2H,m),1.76-1.80 (4H,m),1.52-1.59(2H,m),1.32-1.38(2H,m),1.06(3H,s),0.97 (3H,d,J=7.0Hz),0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com