Synthesis method of 2-ethyl-1-fluoro-4-nitrobenzene
A synthesis method and technology of nitrobenzene, which are applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve problems such as no problems, and achieve the effects of easy operation, high yield, and easy popularization and use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
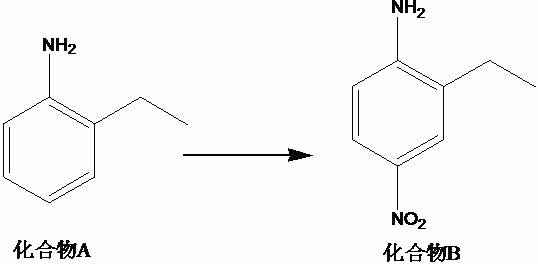
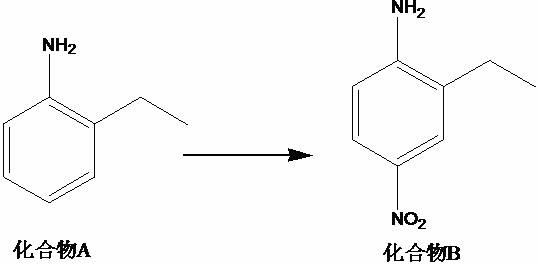
[0015] A kind of synthetic method of 2-ethyl-1-fluoro-4-nitrobenzene, this synthetic method comprises the steps:
[0016] (1) Take the material according to the mass ratio of compound A, concentrated sulfuric acid, and nitric acid solution with a mass fraction of 65% of 1:7 to 10:1, add concentrated sulfuric acid to the reactor, cool to 0°C, and add compound A , stir, then add a nitric acid solution with a mass fraction of 65%, keep warm, rise to 30-35°C, stir, compound B;
[0017] (2) According to compound B, the mass ratio of sodium nitrite is 2:1, the mass fraction is 50% fluoboric acid solution, and the volume ratio of p-xylene is 1:1, the mass fraction is 50% fluoboric acid solution and sodium nitrite The liquid-solid mL / g ratio is 40:1, take the material, put compound B and 50% fluoroboric acid solution into the reactor, cool down to 0°C, add sodium nitrite, stir the reaction, filter, collect For the filter cake, add p-xylene into the reactor, heat to 120-130°C, add the...
Embodiment 1
[0019] A kind of synthetic method of 2-ethyl-1-fluoro-4-nitrobenzene, this synthetic method comprises the steps:
[0020] (1) Add 140g of concentrated sulfuric acid to the reactor, cool to 0°C, add 20g of compound A, stir for 20min, then add 80g of nitric acid solution with a mass fraction of 65%, keep it warm for 1h, rise to 30°C, stir for 15h, and obtain TLC Detection, the reaction of the raw materials is completed, the reaction solution is poured into ice water, the pH is adjusted to 8-9 with 20% aqueous sodium hydroxide solution, 800 mL of ethyl acetate is added, filtered with diatomaceous earth, the liquid is separated, and the aqueous phase is extracted with ethyl acetate (400 mL ), combined the organic phase, mixed the sample with silica gel and passed the column, mobile phase: n-hexane / ethyl acetate=5 / 1, collected the target product, concentrated to obtain 26.3g yellow solid, and obtained compound B with a yield of 95.9% and a purity of 98.3 %;
[0021] (2) Put 20g of...
Embodiment 2
[0024] A kind of synthetic method of 2-ethyl-1-fluoro-4-nitrobenzene, this synthetic method comprises the steps:
[0025] (1) Add 160g of concentrated sulfuric acid to the reactor, cool to 0°C, add 20g of compound A, stir for 20min, then add 80g of nitric acid solution with a mass fraction of 65%, keep it warm for 1h, rise to 33°C, stir for 15h, and obtain TLC Detection, the reaction of the raw materials is completed, the reaction solution is poured into ice water, the pH is adjusted to 8-9 with 20% aqueous sodium hydroxide solution, 800 mL of ethyl acetate is added, filtered with diatomaceous earth, the liquid is separated, and the aqueous phase is extracted with ethyl acetate (400 mL ), combined the organic phase, mixed the sample with silica gel and passed the column, mobile phase: n-hexane / ethyl acetate=5 / 1, collected the target product, concentrated to obtain 26.5 yellow solids, and obtained compound B with a yield of 96.6%;
[0026] (2) Put 20g of compound B and 400mL of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com