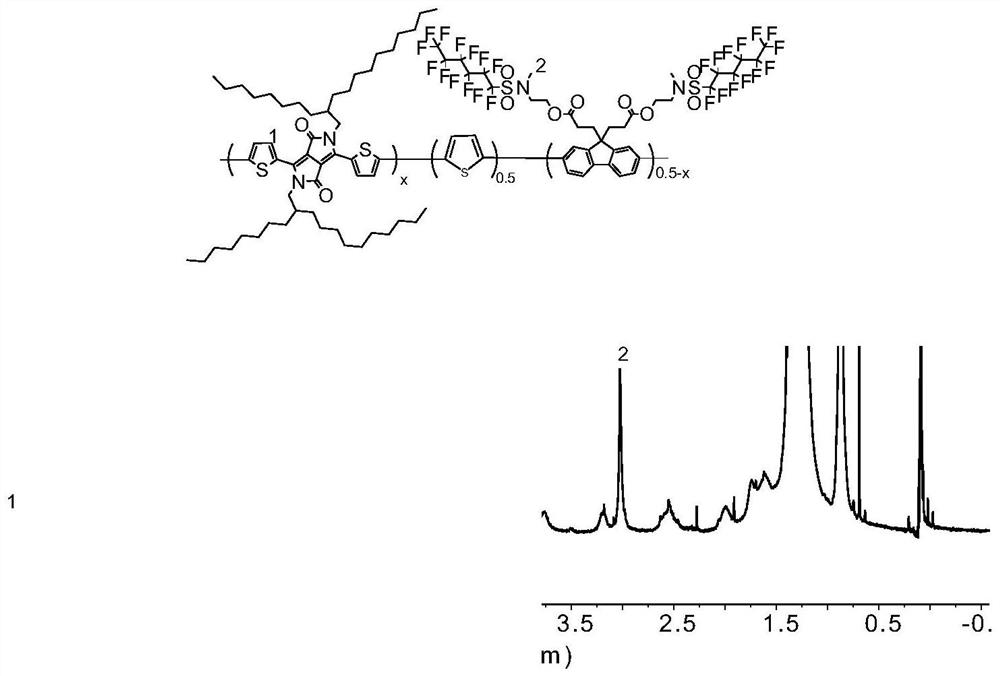
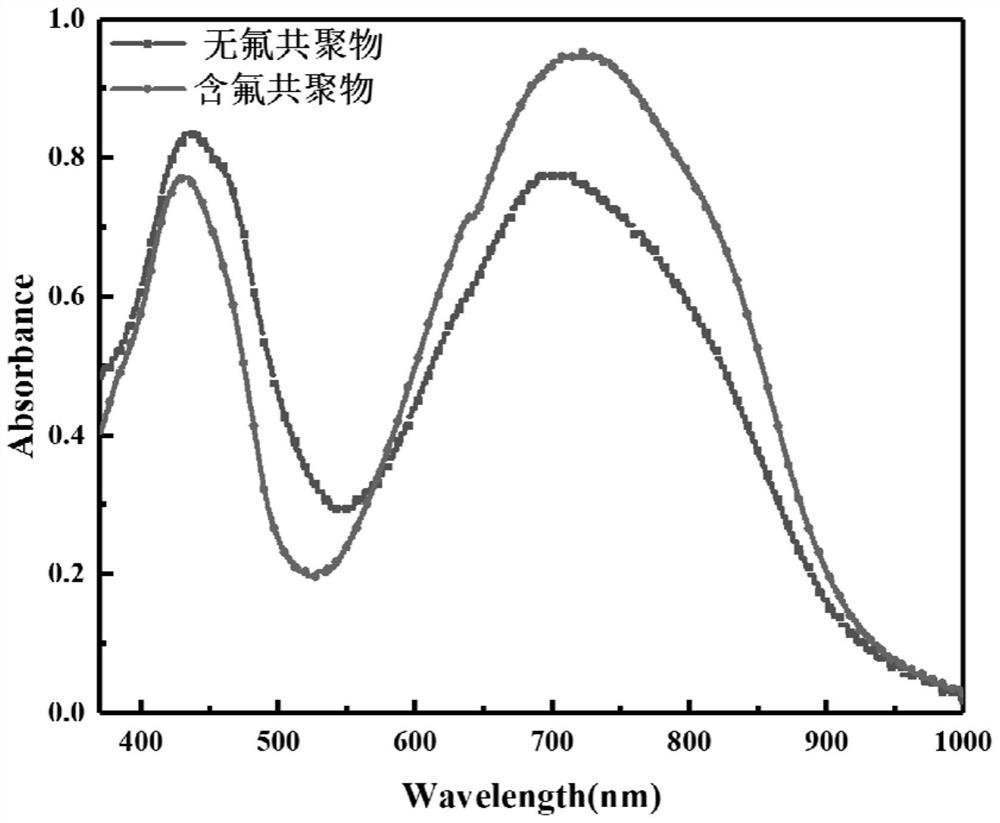
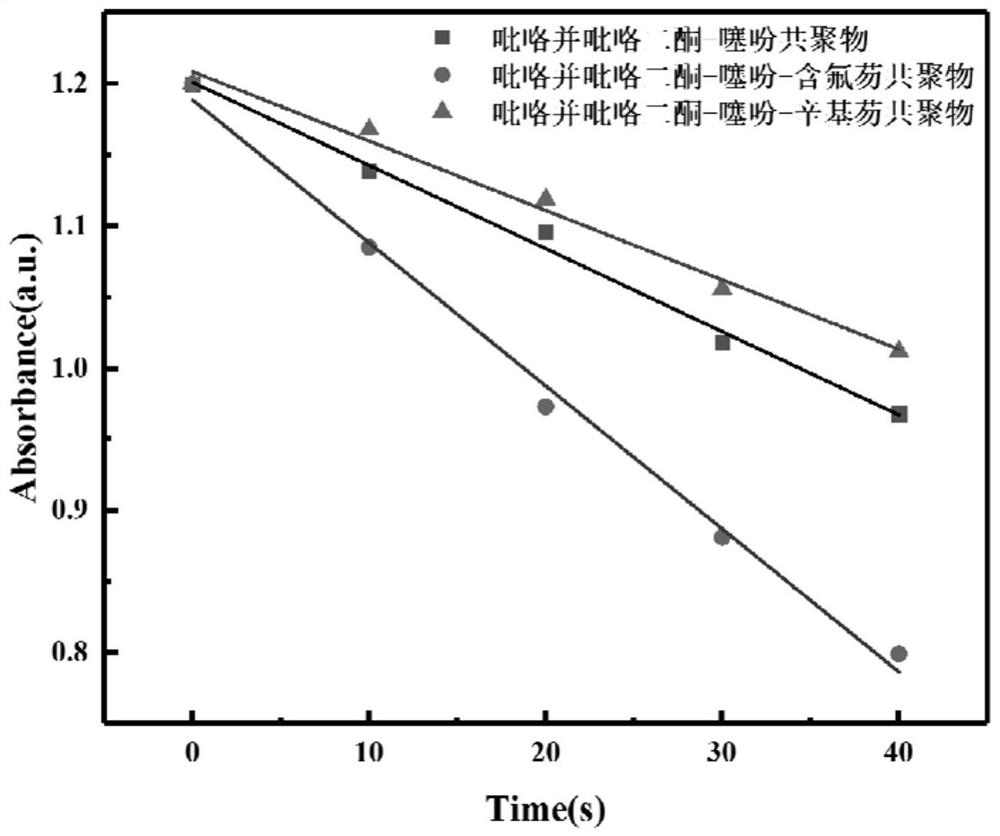
Fluorine-containing near-infrared absorption conjugated polymer and preparation method thereof
A conjugated polymer, near-infrared technology, applied in the field of functional materials, can solve the problems affecting the singlet oxygen efficiency of photosensitizers, the introduction of perfluorinated segments, etc., and achieve the effect of increasing photodynamic therapy efficiency and high singlet oxygen efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Synthesis of 3,6-bis(2-thienyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione
[0050] Add 70mmol potassium tert-butoxide and 40mL tert-amyl alcohol into a 250mL three-necked flask, raise the temperature to 110°C under an argon atmosphere, and stir until the potassium tert-butoxide is completely dissolved. Then, 20 mmol dimethyl succinate was slowly added dropwise, and the reaction was continued for 1 h after the dropwise addition was completed. Then, 50 mmol 2-cyanothiophene was slowly added dropwise, and the reaction was continued for 6 h after the dropwise addition was completed. After the reaction, the reaction solution was poured into a mixed solution of 80 mL of anhydrous methanol, 60 g of ice water and 7 mL of concentrated hydrochloric acid and stirred for 30 min. Filter, wash with water, and dry to obtain a red solid with a yield of 75%.
[0051] (2) Synthesis of 2,5-bis(2-octyldodecyl)-6-(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4-dione
[0052] Add 8mmol 3,6-...
Embodiment 2
[0060] (1) Synthesis of 3,6-bis(2-thienyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione
[0061] Add 70mmol potassium tert-butoxide and 40mL tert-amyl alcohol into a 250mL three-necked flask, raise the temperature to 110°C under an argon atmosphere, and stir until the potassium tert-butoxide is completely dissolved. Then, 20 mmol dimethyl succinate was slowly added dropwise, and the reaction was continued for 1 h after the dropwise addition was completed. Then, 50 mmol 2-cyanothiophene was slowly added dropwise, and the reaction was continued for 6 h after the dropwise addition was completed. After the reaction, the reaction solution was poured into a mixed solution of 80 mL of anhydrous methanol, 60 g of ice water and 7 mL of concentrated hydrochloric acid and stirred for 30 min. Filter, wash with water, and dry to obtain a red solid with a yield of 75%.
[0062] (2) Synthesis of 2,5-bis(2-hexyldecyl)-6-(thien-2-yl)pyrrolo[3,4-c]pyrrole-1,4-dione
[0063]Add 8mmol 3,6-di(2-t...
Embodiment 3
[0071] (1) Synthesis of 3,6-bis(2-thienyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione
[0072] Add 70mmol potassium tert-butoxide and 40mL tert-amyl alcohol into a 250mL three-necked flask, raise the temperature to 110°C under an argon atmosphere, and stir until the potassium tert-butoxide is completely dissolved. Then, 20 mmol dimethyl succinate was slowly added dropwise, and the reaction was continued for 1 h after the dropwise addition was completed. Then, 50 mmol 2-cyanothiophene was slowly added dropwise, and the reaction was continued for 6 h after the dropwise addition was completed. After the reaction, the reaction solution was poured into a mixed solution of 80 mL of anhydrous methanol, 60 g of ice water and 7 mL of concentrated hydrochloric acid and stirred for 30 min. Filter, wash with water, and dry to obtain a red solid with a yield of 75%.
[0073] (2) Synthesis of 2,5-di(dodecyl)-6-(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4-dione
[0074] Add 8mmol 3,6-di(2-thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com