Curcumin analogue, preparation method and application of curcumin analogue in medicine for resisting cancer cell proliferation
A curcumin analogue, cell proliferation technology, applied in chemical instruments and methods, anti-tumor drugs, drug combinations, etc., can solve low water solubility, rapid metabolism, low bioavailability, low anti-cancer effect, curcumin pharmacokinetics Problems such as poor kinetic properties, to achieve the effect of preventing cancer cell metastasis, inhibiting cell colony formation, inhibiting cell migration and invasion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7
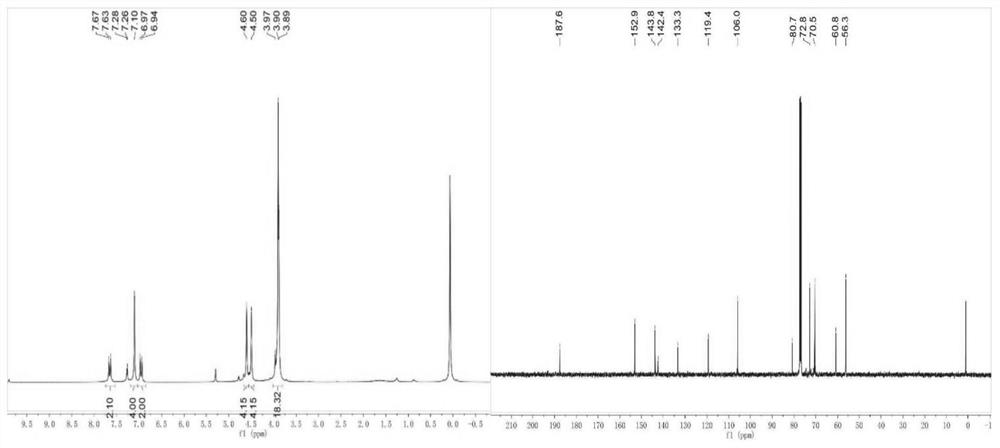
[0058] A kind of curcumin analogue, its structural formula is shown in following general formula A, comprises the structure shown in any one of general formula A1-A7 in table 3, comprises following preparation steps:
[0059] (1) Preparation of 1,1'-ferrocene dicarbaldehyde: First, n-butyllithium solution (67ml, 1.6M, 0.10mol) was added dropwise to 200ml containing ferrocene (10g, 0.054mol) at room temperature and tetramethylethylenediamine (TMEDA) (14.7g, 0.25mol) in anhydrous hexane suspension; the mixed solution was stirred at room temperature for 18 hours, and an orange dilithiated ferrocene precipitate was precipitated. The liquid was cooled to 0°C, dimethylformamide (DMF) (7.85g, 0.11mol, 8.3ml) was added to the suspension, and it was stirred at room temperature for 2 hours for Bouveault aldehyde synthesis reaction; then precooled The reaction was quenched with hydrochloric acid (200ml, 5M), the mixture was extracted three times with ethyl acetate (100ml), the ethyl acet...
Embodiment 8-20
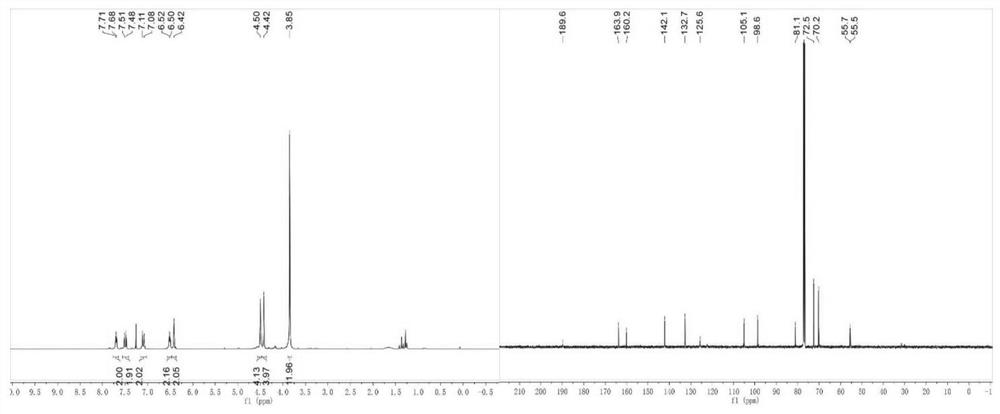
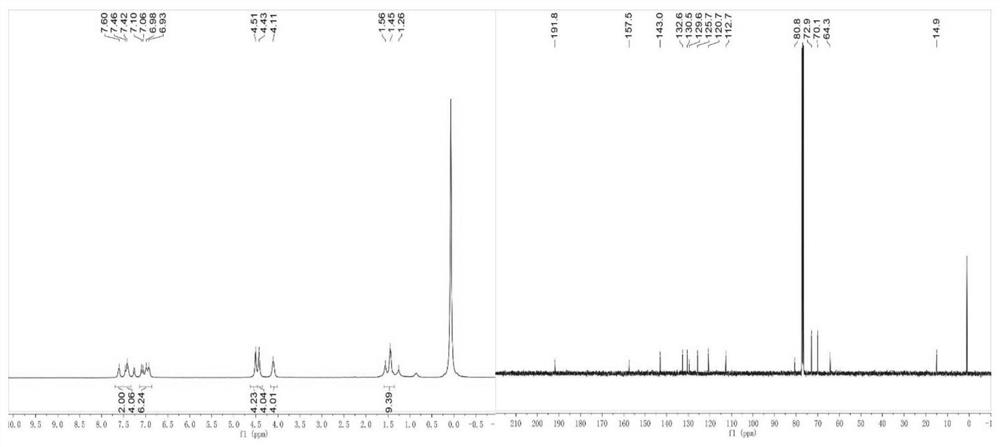
[0076] A curcumin analog, shown in its structural formula B, including the structure shown in any of the general formulas B1-B13 in Table 3, including the following preparation steps:
[0077] (1) Preparation of benzylidene acetone derivative: Benzaldehyde derivative (80.7 mmol), acetone (74 ml) and water (34 ml) were mixed well, followed by NaOH solution (30.3 mmol, 5.5 ml) and water (303 ml) Add to the solution to obtain a yellow mixture; the yellow mixture is stirred at room temperature for 20 hours, the organic layer is collected and concentrated in vacuo to give the corresponding benzylidene acetone derivative.
[0078] (2) Preparation of curcumin analog compound: 1,1'-Ferrocene dicarbaldehyde (0.5 mmol) and the above benzylidene acetone derivative (1 mmol) were dissolved in methanol (30 ml), and NaOH solution (1.5 mmol) was added. M, 5 ml), stirred at room temperature for 24 hours; the mixture was concentrated to dryness under vacuum, and the product was purified by colu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com