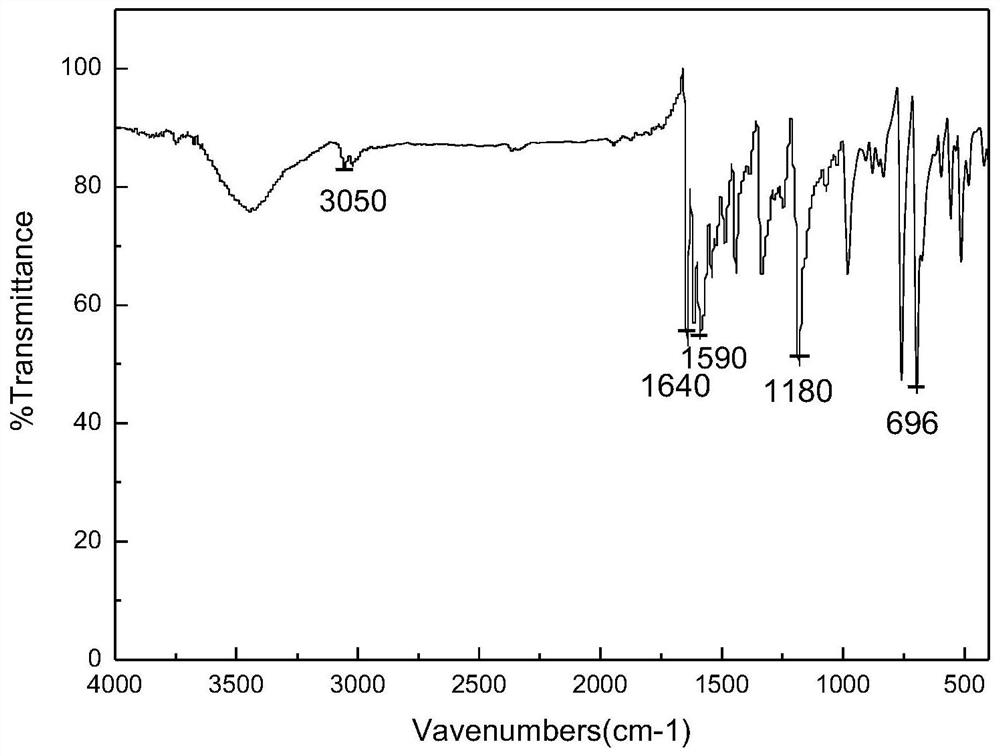
A kind of preparation method of three (dibenzylideneacetone) dipalladium (0)
A technology of dibenzylideneacetone and palladium dichloride is applied in chemical instruments and methods, compounds containing elements of Group 8/9/10/18 of the periodic table, palladium organic compounds, etc. The problems such as the amount of added dibenzylideneacetone, long reaction time, and difficult operation are solved, and the effects of short reaction time, simple preparation method and simple equipment are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1.1 Preparation of palladium dichloride:
[0021] Add nitric acid with a mass concentration of 65% to high-purity palladium powder with a mass purity of not less than 99.95%. The quality of nitric acid is 0.7 times that of the palladium powder. After heating and waiting for its reaction, slowly add hydrochloric acid with a mass concentration of 36%. It is 2.1 times the mass of palladium powder. After the palladium powder is completely dissolved, a small amount of hydrochloric acid with a mass concentration of 36% is added several times to completely replace the nitric acid in the solution. If the particle size is less than 10 μm, palladium dichloride with the desired mass purity of 99.9% can be obtained.
[0022] 1.2 Synthesis of dibenzylideneacetone:
[0023] Measure 10L mass concentration of 10% sodium hydroxide solution and 8L mass concentration of 95% absolute ethanol in a 30L glass reactor, add 10.5L benzaldehyde and 0.36L acetone successively under mechanical sti...
Embodiment 2
[0025] Preparation of tris(dibenzylideneacetone)dipalladium(0):
[0026] Step 1. Under a nitrogen atmosphere, add 32 L of absolute ethanol and 0.85 kg of anhydrous sodium acetate into a glass reactor heated by a water bath. When the system temperature is heated to 80° C., add 3 kg of dibenzylidene acetone obtained in Example 1, and stir After reacting for 20 minutes, add ice to the water-bath heater to cool the system down to 45°C rapidly, then add 1kg of palladium dichloride prepared in Example 1, react at 45°C for 3h, and filter through a funnel to obtain bis(dibenzylideneacetone ) Palladium(0);
[0027] Step 2. Under a nitrogen atmosphere, add 5 L of acetone to a glass reactor heated by a water bath. After the temperature of the acetone in the reactor is heated to 30° C., add bis(dibenzylideneacetone) palladium (0) obtained in Step 1, 30 React at ℃ for 3 hours, filter, wash with deionized water and absolute ethanol, and dry in an oven at 40℃ to obtain the product tris(dibe...
Embodiment 3
[0030] Preparation of tris(dibenzylideneacetone)dipalladium(0):
[0031] Step 1. Under a nitrogen atmosphere, add 18 L of absolute ethanol and 0.48 kg of anhydrous sodium acetate into a glass reactor heated by a water bath, and add 0.86 kg of dibenzylidene acetone obtained in Example 1 when the system temperature is heated to 60° C. After stirring and reacting for 40 minutes, add ice to the water bath heater to rapidly cool the system to 57° C., then add 0.42 kg of palladium dichloride prepared in Example 1, react for 1.5 hours at 57° C., and filter through a funnel to obtain bis(dimethoxymide) benzylacetone) palladium (0);
[0032] Step 2. Under a nitrogen atmosphere, add 2.5 L of acetone to a glass reactor heated by a water bath. After the temperature of the acetone in the reactor is heated to 15° C., add bis(dibenzylideneacetone) palladium (0) obtained in Step 1, Heat the reaction for 1 h, filter, wash with deionized water and absolute ethanol, and dry in an oven at 30°C t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com