Bosentan solid pharmaceutical composition and preparation method thereof
A composition and technology of bosentan, applied in the field of bosentan pharmaceutical composition and its preparation, can solve problems such as low dissolution rate and difficulty in medication for children
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
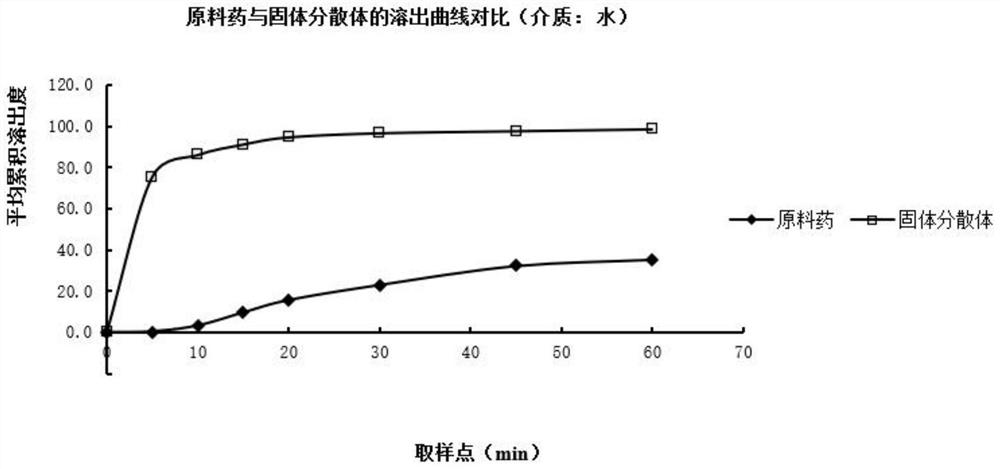
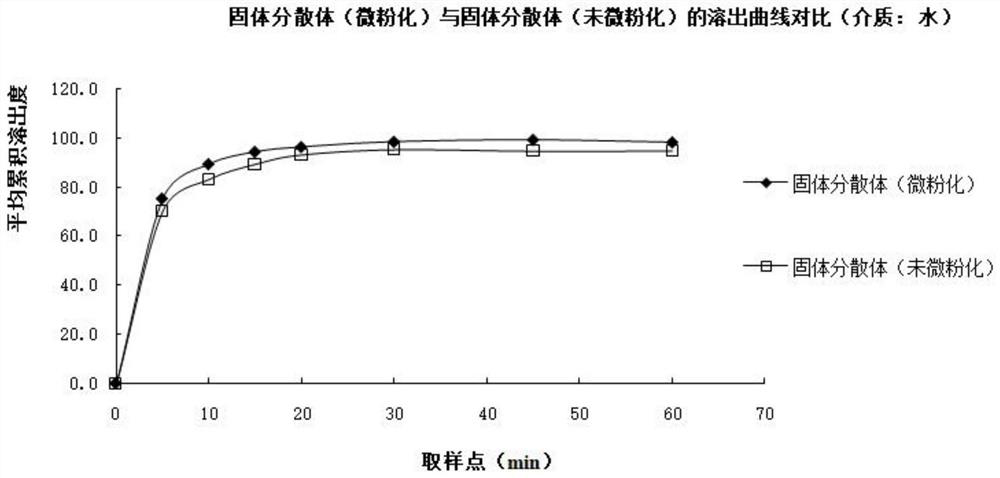
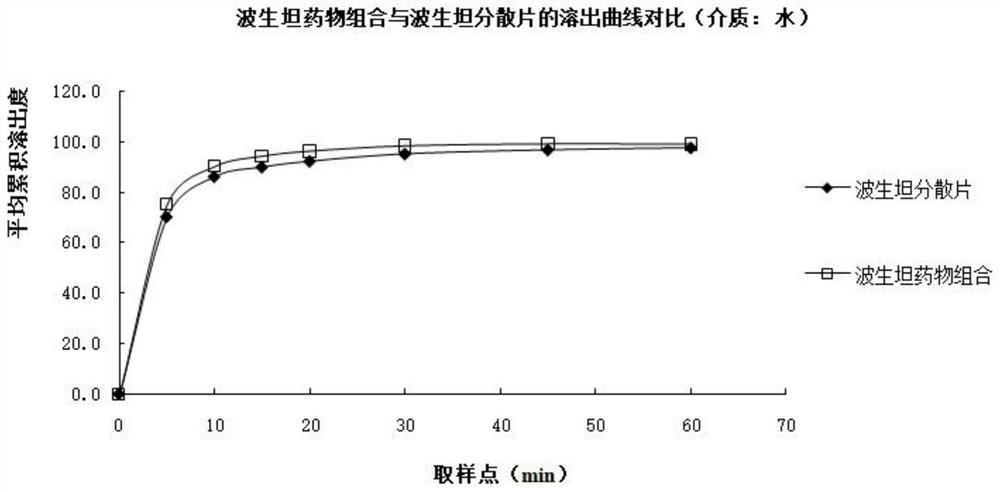
Image
Examples
example 1
[0056] Using the jet pulverization method, the bosentan raw material is subjected to high-speed shear pulverization by a pulverizer, and then added to an air pulverizer for ultra-fine pulverization to obtain micronized bosentan, and the final particle size is controlled to be 10nm-20nm.
example 2
[0058] Prescribing information is as follows:
[0059]
[0060] The preparation method is as follows:
[0061] 1) Mix the prescribed amount of bosentan with PPVVA64 for 10 to 15 minutes, pass through a 40-mesh sieve for later use;
[0062] 2) Use a hot melt extruder, preheat at 130-160°C, add the mixed powder in step 1) after stabilization, adjust the feeding speed to 50-150 RPM, collect the solid dispersion, pass through an 80-mesh sieve after pulverization, and set aside;
[0063] 3) Lactose, microcrystalline cellulose, sodium saccharin, xanthan gum, sodium citrate and orange essence of recipe quantity are mixed after 10 minutes, add magnesium stearate and mix for 3 minutes to obtain mixed powder;
[0064] 4) dry granulating the mixed powder of step 3), pressure 5~8MPa, feed rotation speed 2RPM, roller rotation speed 1RPM, pass through an 18-mesh sieve after pulverization, and collect the granular powder;
[0065] 5) Mix the solid dispersion of step 2) with the granular...
example 3
[0067] Prescribing information is as follows:
[0068]
[0069]
[0070] The preparation method is as follows:
[0071] 1) Mix the prescribed amount of bosentan with PPVVA64 for 10 to 15 minutes, pass through a 40-mesh sieve for later use;
[0072] 2) Use a hot melt extruder, preheat at 130-160°C, add the mixed powder in step 1) after stabilization, adjust the feeding speed to 50-150 RPM, collect the solid dispersion, pass through an 80-mesh sieve after pulverization, and set aside;
[0073] 3) Mix the lactose, microcrystalline cellulose, sodium saccharin, xanthan gum, sodium dihydrogen phosphate, orange essence and the solid dispersion of step 2) in the recipe amount for 10 to 15 minutes, then add talc and mix for 3 minutes to obtain mixed powder;
[0074] 4) The solid dispersion of step 2) and the mixed powder of step 3) are mixed for 10 to 15 minutes, and then the mixture is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com