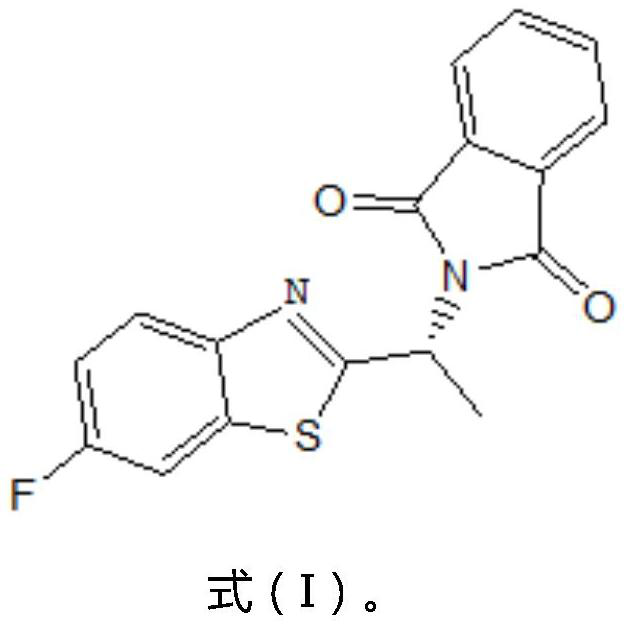
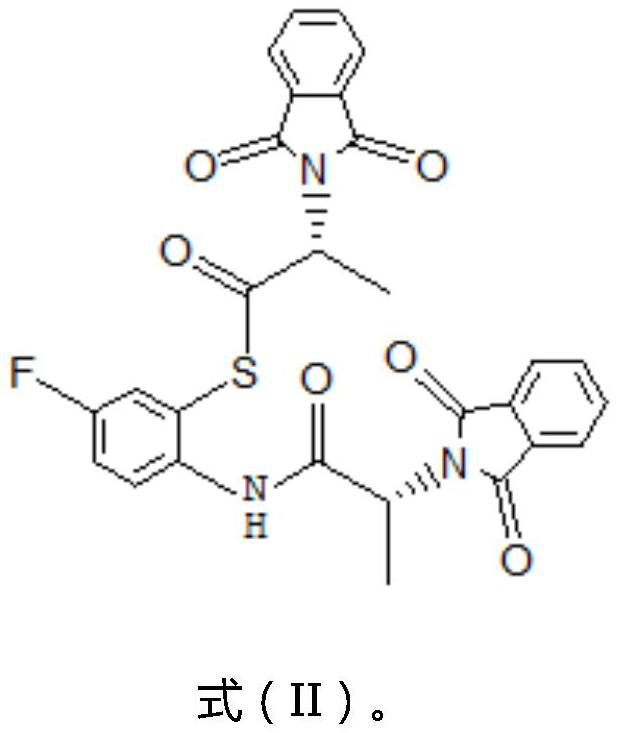
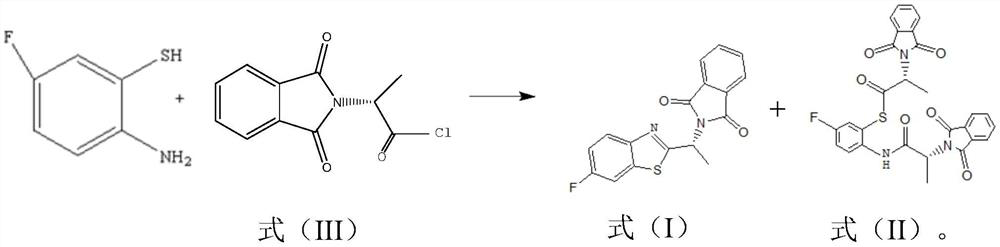
Preparation method of (R)-1-(6-fluoro-2-benzothiazolyl)-ethylamine and preparation intermediate of (R)-1-(6-fluoro-2-benzothiazolyl)-ethylamine
A technology for intermediates and ethylamines, applied in the field of preparing -1--ethylamine salts, can solve the problems of low yield, high price, complicated post-processing and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]Add 240g of toluene, 40g of D-alanine, 68g of phthalic anhydride and 1g of triethylamine into the reaction flask, heat and reflux to separate water, react for 2.5-3.5h, add 66g of thionyl chloride dropwise, and control the dropping rate to Keep the temperature in the reaction bottle at 60-65°C. After dropping, raise the temperature to 77°C. After reacting for 5 hours, monitor and confirm the completion of the reaction by thin-layer chromatography, then concentrate at -0.06MPa and 55°C for 2 hours to obtain the formula (III) 106 g of the indicated acid chloride compound.
[0046] Under nitrogen protection, 43g of 2-amino-5-fluorothiophenol was dissolved in 128g of water, and the aqueous solution was added dropwise to 510g of hydrochloric acid with a concentration of 36wt%. After completion, 106 g of the acid chloride compound represented by the formula (III) prepared above was added dropwise, and the rate of addition was controlled so that the temperature was controlled a...
Embodiment 2
[0057] Add 635g of toluene, 61.5g of D-alanine, 102g of phthalic anhydride and 1g of triethylamine into the reaction flask, heat and reflux to separate water, react for 2.5-3.5h, add 82g of thionyl chloride dropwise, and control the dropping rate To keep the temperature in the reaction bottle at 60-65°C, after dropping, raise the temperature to 72°C, react for 5 hours, monitor and confirm the completion of the reaction by thin-layer chromatography, concentrate at -0.09MPa, 60°C for 1 hour, and obtain the formula (III) 164 g of the indicated acid chloride compound.
[0058] Under the protection of nitrogen, 43g of 2-amino-5-fluorothiophenol was dissolved in 85g of water, and the solution was added dropwise to 310g of hydrochloric acid with a concentration of 35wt%, and the dropping rate was controlled so that the temperature was always controlled at 0-10°C. After dropping, add 164 g of the acid chloride compound represented by the above-mentioned formula (III) dropwise, control...
Embodiment 3
[0061] Put 80.0 g of the compound of formula (I) into the reaction flask, add 1276 g of methanol aqueous solution with a concentration of 55 wt %, and 55 g of hydrazine hydrate, and conduct hydrazinolysis reaction at 45-50 ° C for 2.5-3.5 h under the protection of nitrogen, and then add hydrochloric acid to adjust to The pH is acidic, stirred for 1 h, cooled to below 15°C and filtered after the reaction, added 115 g of p-toluenesulfonic acid solution with a concentration of 50 wt % to the filtrate, cooled to 3°C to crystallize for 1.5 h, filtered to obtain the compound of formula (IV) 71.0 g of p-toluenesulfonate salt of (R)-1-(6-fluoro-2-benzothiazolyl)-ethylamine.
[0062] Add the above-prepared (R)-1-(6-fluoro-2-benzothiazolyl)-ethylamine methanesulfonate to an equal mass of water, adjust the pH to 10-12 with sodium hydroxide to dissolve the target compound , extract with dichloromethane, separate the layers, and concentrate the organic phase to obtain the compound of formu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com