Fusion protein containing interferon alpha, recombinant strain for expressing fusion protein and preparation method of fusion protein
A fusion protein, interferon alpha technology, applied in the field of biomedicine, can solve problems such as reducing the activity of interferon alpha, and achieve the effects of blocking immune escape, improving the effect of virus clearance, and reducing morbidity and mortality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 A fusion protein containing interferon alpha, a recombinant strain expressing the fusion protein and its preparation method
[0039] 1. Insert the fusion gene fragment rpoALB-nsp5 site-IFNα into the secretion expression vector pPICZαA vector
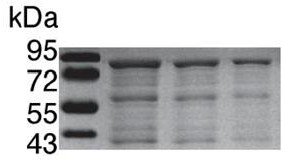
[0040] Suggestions on the fusion protein module of rpoALB-nsp5 site-IFNα figure 1 (See appendix SEQ ID NO. 1 for the complete nucleic acid sequence and SEQ ID NO. 2 for the amino acid sequence).
[0041] Nucleic acid sequences of each module of the fusion protein of rpoALB-nsp5 site-IFNα
[0042] (1) rpoALB nucleic acid sequence (SEQ ID NO.3)
[0043] (3) nsp5 site nucleic acid sequence (SEQ ID NO.4)
[0044] (4) IFNα nucleic acid sequence (SEQ ID NO. 5).
[0045]According to the nucleic acid sequence of rpoALB, the nucleic acid sequence of nsp5 site, and the nucleic acid sequence of IFNα, Sangon Bioengineering (Shanghai) Co., Ltd. was entrusted to synthesize the entire expression cassette, and inserted into the ...
Embodiment 2
[0068] Example 2 Plasma stability detection of rpoALB-nsp5 site-IFNα
[0069] Blood was collected from healthy BALB / c mice, centrifuged to collect plasma, diluted with PBS to 50% plasma (V / V). 8) Add 50% plasma to a final concentration of 10µg / mL, incubate at 37°C for 0, 0.25, 0.5, 1.0, 3.0, 6.0, 12 and 24h, take 100µl each, centrifuge at 12000g for 30min, and collect the supernatant. According to the "2017 Version of Veterinary Drug Quality Standards", the MDBK-VSV detection system and the cytopathic inhibition method were used to detect the fusion protein activity. The results showed that the time for 50% decrease of IFNα activity was about 6 h, and the time for rpoALB-nsp5 site-IFNα activity to decrease by 50% was 24.5 h. For details, see Image 6 .
Embodiment 3
[0070] Example 3 Laboratory control test of rpoALB-nsp5 site-IFNα
[0071] 30 piglets that were born without colostrum were randomly divided into 3 groups, 10 pigs in each group. The first group was the rpoALB-nsp5site-IFNα treatment group; the second group was the IFNα treatment group (see patent CN201911058504.8 for specific preparation standards) ; Group 3 is no treatment group. Piglets in each group were injected with 3 mL of PED virus solution (the virus concentration was 1 × 10 6 TCID50 / mL), then immediately, the piglets of the experimental group were intramuscularly injected with the purified rpoALB-nsp5 site-IFNα obtained in Example 1 (the antiviral activity was 1 × 10 6 IU / mL), 2 ml each time, once a day for 3 consecutive days; the piglets in the second group were intramuscularly injected with IFNα ((the antiviral activity was 1 × 10 6 IU / mL), 2 ml each time, once a day for 3 consecutive days; piglets in the blank control group were intramuscularly injected with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com