Annular Gd (III) complex as well as preparation method and application thereof
A complex and ring-type technology, which can be used in pharmaceutical formulations, preparations for in vivo tests, organic chemistry, etc., can solve the problems of polymetallic gadolinium residues, poor stability, and poor targeting, and achieve improved targeting and stability. Sexual enhancement, high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
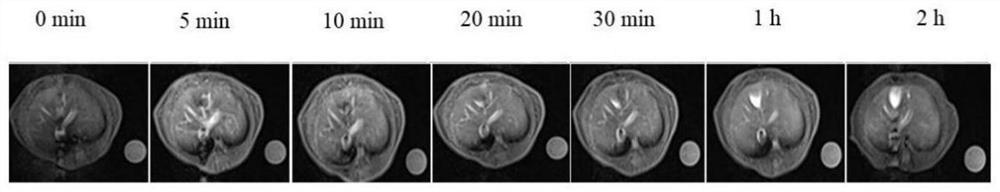
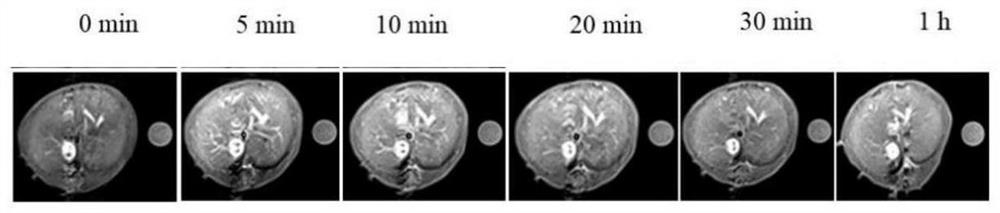
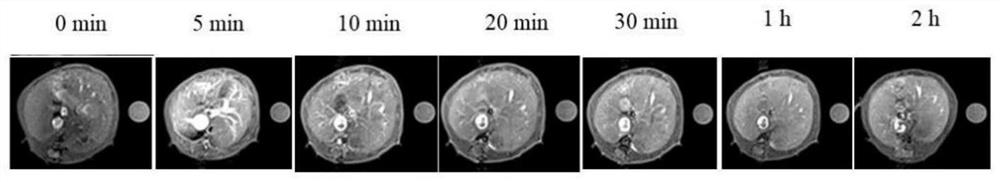
Image
Examples
preparation example Construction
[0081] In the present invention, in the formula I, R is H, R' is H, and R" is When, the preparation method of the cyclic Gd (III) complex comprises the following steps:
[0082] (1) In a nitrogen atmosphere, mix a compound having a structure shown in Formula A-1, DO3A, potassium carbonate and acetonitrile, and carry out an affinity substitution reaction to obtain a compound having a structure shown in Formula A-2;
[0083] (2) Mix the compound having the structure shown in formula A-2 obtained in the step (1) with an aqueous solution of tetrahydrofuran, methanol and lithium hydroxide, and then perform a hydrolysis reaction to obtain a first reaction precursor;
[0084] (3) In a nitrogen atmosphere, the first reaction precursor obtained in step (2) is mixed with HATU, dichloromethane, amine and DIPEA and then subjected to a condensation reaction to obtain a compound having a structure shown in formula A-3;
[0085] (4) Mix the compound having the structure shown in formula ...
Embodiment 1
[0251] A cyclic Gd(III) complex having a chemical structure shown in formula I, in which R is H, R' is H, and R" is Denoted as complex GdL1.
[0252] The preparation method is:
[0253]
[0254] (1) The compound (1.0g, 3mmol) with the structure shown in formula A-1 and DO3A (1.6g, 3mmol), potassium carbonate (0.6g, 6mmol) were dissolved in 20mL acetonitrile, and heated to Stir at 70°C for nucleophilic substitution reaction for 16 hours, concentrate the product of nucleophilic substitution reaction and purify the resulting mixture by silica gel column chromatography (ethyl acetate:methanol=5:1) to obtain a compound with the structure shown in formula A-2 , yield 76%, 1 HNMR (400MHz, CDCl3) δ (ppm): 8.00 (d, 2H, J = 8.40Hz), 7.54 (d, 2H, J = 8.40Hz), 4.52 (s, 1H), 3.92 (s, 3H), 3.33 (s,2H),3.18(s,3H),2.80(m,15H),1.48(s,20H),1.43(s,16H). 13 C NMR (100MHz, CDCl3) δ (ppm) 171.26, 171.09, 170.86, 166.93, 143.16, 129.46, 129.38, 129.10, 81.65, 80.81, 77.41, 77.09, 76.77, 69.45,...
Embodiment 2
[0260] A cyclic Gd(III) complex having a chemical structure shown in formula I, in which R is H, R' is H, and R" is The compound is denoted as complex GdL2.
[0261] Preparation:
[0262]
[0263] Prepare complex GdL2 according to the method for embodiment 1, HPLC-MS (ESI - ) calculated for C 38 h 43 GdN 5 o 9 ,[M] = 871.23, found 871.25;
[0264] The difference from Example 1 is that in the step (2), p-ethoxybenzylamine is replaced by 3,3-diphenylpropylamine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com