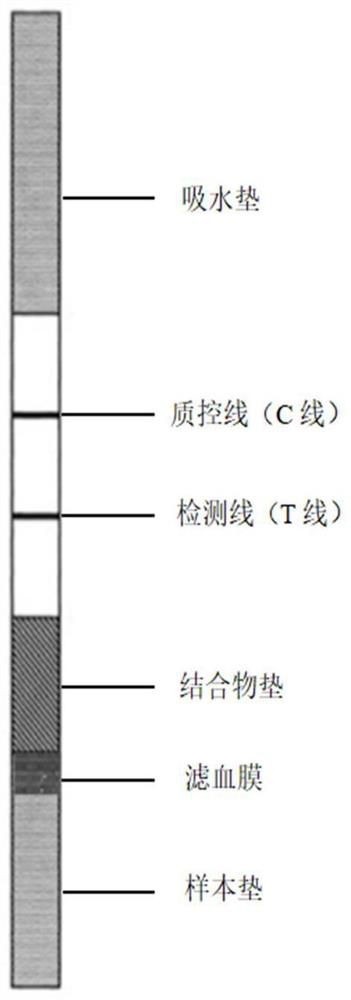
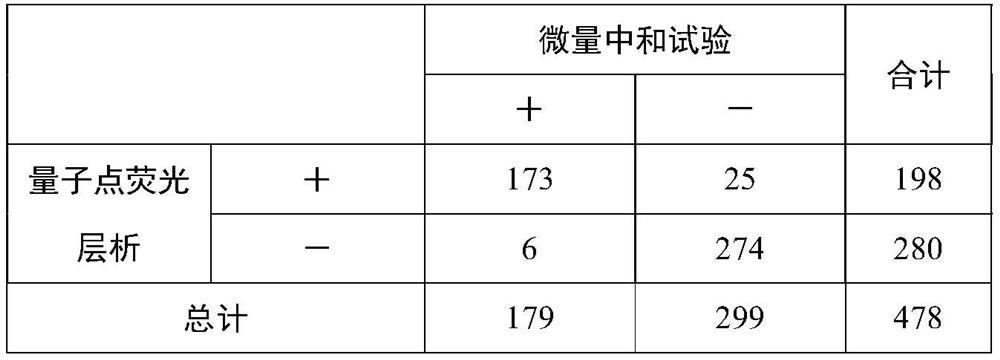
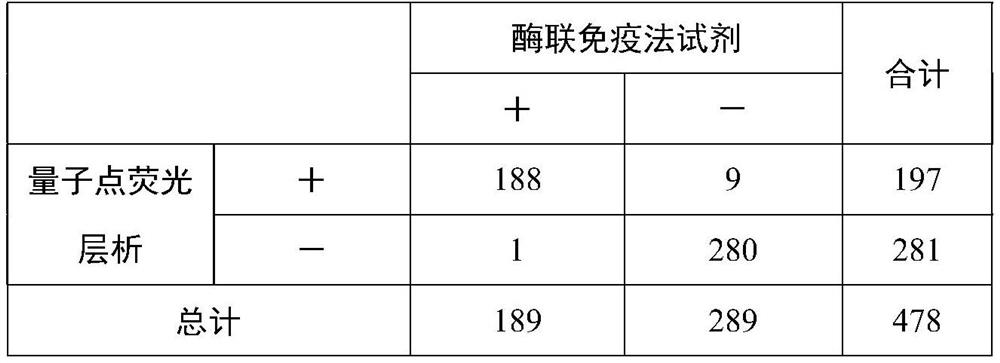
Fluorescence immunochromatography test strip and preparation method thereof
A technology of fluorescent immunochromatography and test strips, which is applied in the field of immunoassay, can solve problems such as difficult to effectively meet rapid and large-scale detection, high detection condition requirements, and high biosafety risk, so as to improve detection stability and biosafety Low risk, effect of improving binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] 1. Preparation of antigen-quantum dot fluorescent microsphere complexes
[0080] Use 50mM HEPES solution with pH 7.0 to dilute the quantum dot fluorescent microspheres at a volume ratio of 9:1 to obtain a quantum dot fluorescent microsphere solution, add 1 times the volume of the quantum dot fluorescent microsphere solution with 5% EDC and NHS solution, activate 15 minute. Centrifuge at 14,000 rpm at 4°C for 15 minutes, remove the supernatant, and reconstitute with a 10-fold reconstituted solution of quantum dot fluorescent microsphere solution. Add the novel coronavirus RBD antigen (amino acid sequence shown in SEQ ID NO. 1) according to 1.8mg / mL quantum dot fluorescent microsphere solution, mix for 60 minutes, add 0.5 times the total volume of the above solution with a concentration of 10% BSA solution, Mix well for 120 minutes, centrifuge at 14,000 rpm for 15 minutes at 4°C, remove the supernatant, and reconstitute with a reconstituted solution of 10 times the volum...
experiment example 1
[0100]The novel coronavirus RBD protein used in Example 1 of the present invention is transformed from the reported novel coronavirus RBD protein (the amino acid sequence before the transformation is shown in SEQ ID NO. 2), and this experimental example further compares the transformed novel coronavirus The sensitivity of coronavirus RBD protein and commercially available new coronavirus RBD protein (purchased from Jiangsu Dongkang Biomedical Technology Co., Ltd., product number EMSARS-COV2 RBD01) as antigens, specifically using the novel coronavirus RBD protein involved in Example 1 ( The amino acid sequence is shown in SEQ ID NO.1) and the commercially available novel coronavirus RBD protein is prepared to obtain a fluorescent immunochromatography test strip (the method is exactly the same as that of Example 1), and the results are as follows:
[0101] Table 3 Sensitivity comparison results of different novel coronavirus S protein RBD antigens
[0102] Weak positiv...
experiment example 2
[0104] This experimental example compares the influence of different coating methods on the detection results of fluorescent immunochromatography test strips, specifically for the combination of novel coronavirus RBD antigen-quantum dot complexes and chicken IgY antibody-quantum dot complexes in step 4 in Example 1 The way that the mixture of dot complexes was coated on the sample and the integrated pad of the conjugate was carried out as follows:
[0105] 1. Roll out:
[0106] (1) Clean the screen window, grate, roller, pad, and dry it for later use.
[0107] (2) Dosing: take a suitable clean container and stirrer, add 9ml RBD antigen-quantum dot fluorescent microsphere complex and 3ml chicken IgY antibody-quantum dot fluorescent microsphere complex into the above container, add 78ml complex solution , and mix at medium speed for 5 minutes.
[0108] (3) Take 1 piece of glass fiber, place it on the backing plate, adjust the volume of the 5ml pipette to 4.5ml, press 9ml / sheet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com