Method for preparing organic alcohol by catalyzing ethanol to reduce organic aldehyde compound
A compound and organic aldehyde technology, applied in the field of selective reduction of organic aldehyde compounds, can solve the problems of cumbersome catalyst synthesis process and high cost of large-scale preparation, and achieve the effects of low price, mild reaction, easy regulation, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
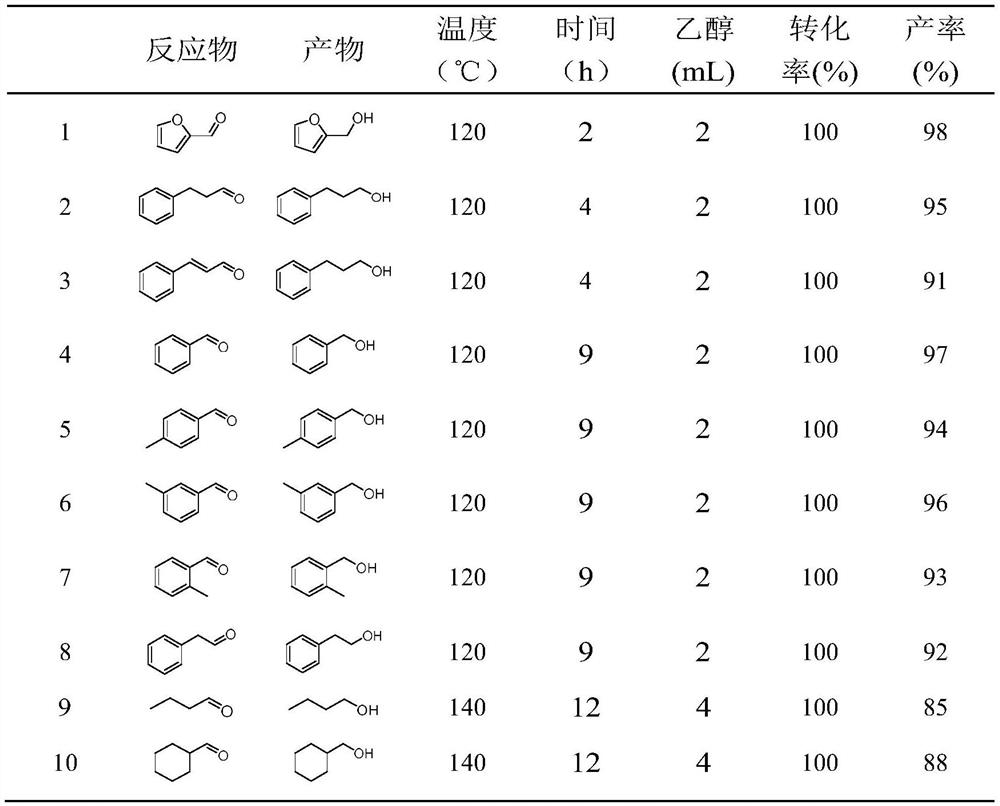
Examples
Embodiment 1
[0027] The CuZnAl skeleton catalyst was prepared by the alkali etching method, which included the following steps: configure 6.25mol / L NaOH aqueous solution, place it in a water bath at 5°C, slowly add 10g of 300-mesh Tiweed alloy (CuZnAl), etch for 0.5h, and react After finishing, rinse with water until it becomes neutral to obtain CuZnAl framework catalyst CuZnAl-0.5, which is stored in absolute ethanol.
Embodiment 2
[0029] 200mg CuZnAl-0.5 (Example 1), 2mL concentration>99% ethanol, 0.5mmol 5-Hydroxymethylfurfural were added to a 35mL pressure reaction tube, and 1bar N was introduced 2 , at 120 ° C with a stirring speed of 700 rpm for 3 h, the reaction product was confirmed by the gas phase that the main product was indeed 2,5-furandimethanol. Using naphthalene as the internal standard, the yield of 2,5-furandimethanol was 92% through quantitative analysis by gas chromatography, and the yield calculation formula was: target product yield (%)=the amount of the target product actually obtained ÷ Amount of theoretical target product×100%.
Embodiment 3
[0031] It is basically the same as Example 2, the difference is: CuZnAl-0.25 with an etching time of 0.25h is used instead of CuZnAl-0.5 in Example 2, and the detection result is that the product of 2,5-furandimethanol is obtained in this example. The rate was 71%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com