Lactobionic acid-novel indocyanine green conjugate as well as preparation method and application thereof
A new indocyanine green and lactobionic acid technology, applied in the field of biomedicine, can solve the problems of poor repeatability, poor tumor photothermal, photodynamic therapy fluorescence imaging of tumors, and complicated drug loading steps, and achieve the effect of simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of 1,6-Hexanediamined Neoindocyanine Green
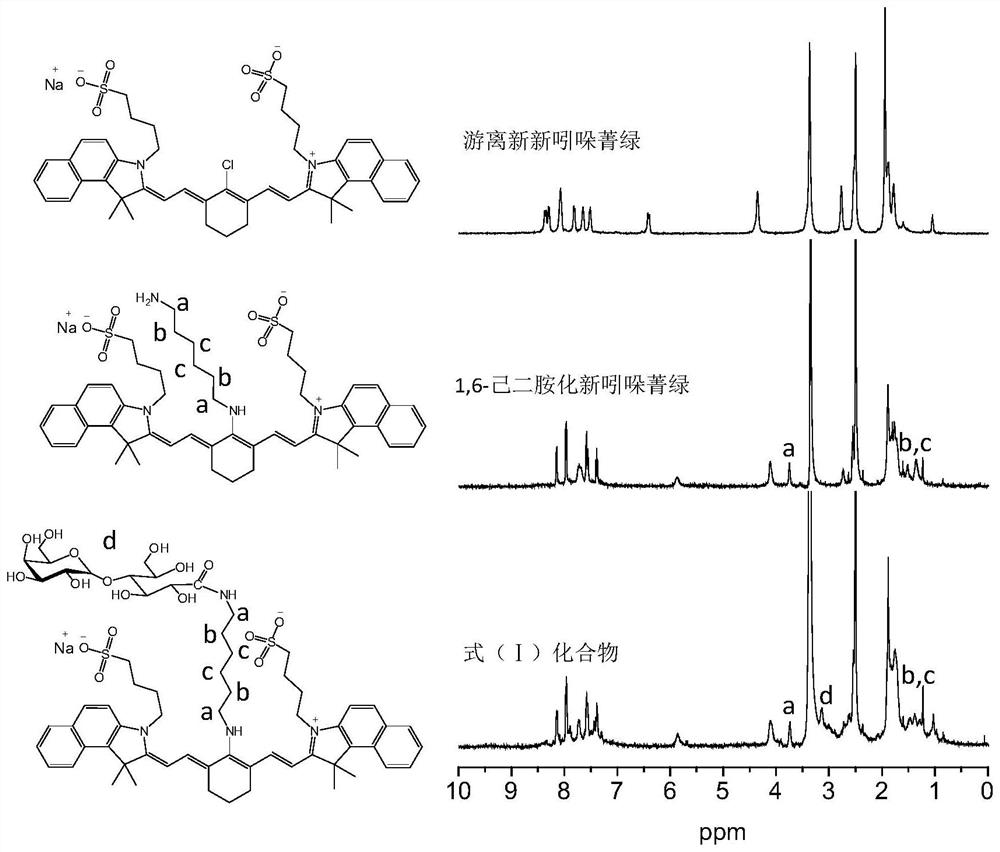
[0044] 20 mL of anhydrous methanol was added to a 50 mL round-bottomed flask, followed by magnetic stirring, neo-indocyanine green (85 mg, 0.1 mmol) and 1,6-hexanediamine (116 mg, 1.0 mmol) were added and stirred at room temperature to dissolve, and heated to 50 Reflux for 3 hours at ℃, then cooled to room temperature, added to a dialysis bag (molecular weight cut-off 800Da), dialyzed in ultrapure water for three days, freeze-dried to obtain 87 mg of blue powdery solid with a yield of 94%.
[0045] The synthesized 1,6-hexanediamined neoindocyanine green was characterized by 1,6-hexanediamined neoindocyanine green by H NMR spectroscopy and high performance liquid chromatography. like figure 1 As shown, compared with the free neo-indocyanine green, the characteristic peak of the methylene group in the 1,6-hexanediamine group appears in the H NMR spectrum of the 1,6-hexanediamined neo-indocyanine green ( ...
Embodiment 2
[0046] Example 2: Preparation of compounds of formula (I)
[0047] Add 10 mL of N,N-dimethylformamide to a 50 mL round-bottomed flask, add magnetic stirring, add lactobionic acid (115 mg, 0.32 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbon Diimine hydrochloride (153 mg, 0.8 mmol), 4-dimethylaminopyridine (20 mg, 0.16 mmol) and 1,6-hexanediamined neoindocyanine green (74 mg, 0.08 mmol) were stirred and dissolved at room temperature, Heated to 50°C for 24 hours, then cooled to room temperature, added to a dialysis bag (molecular weight cut-off 800Da), dialyzed in ultrapure water for three days, freeze-dried, and the freeze-dried product was separated and purified by column chromatography to obtain a blue color Powdered solid 89 mg (purity 93%), yield 88%.
[0048]The synthesized compound of formula (I) was characterized by H NMR spectroscopy, infrared spectroscopy and high performance liquid chromatography. like figure 1 As shown, compared with 1,6-hexanediamined neoindocyani...
Embodiment 3
[0049] Example 3: Preparation of cystamined neoindocyanine green
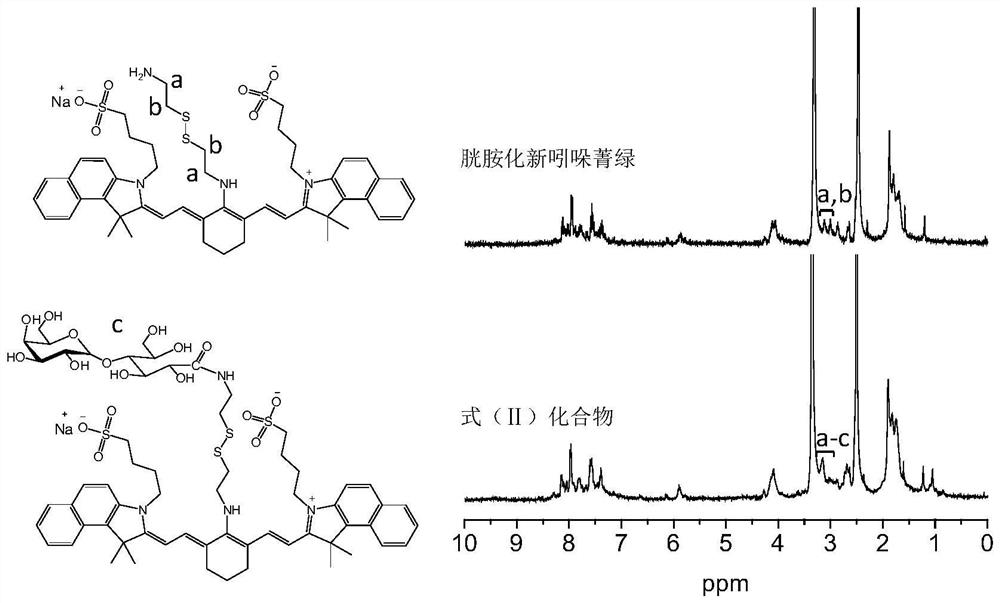
[0050] 20 mL of anhydrous methanol was added to a 50 mL round-bottomed flask, followed by magnetic stirring, and neo indocyanine green (85 mg, 0.1 mmol), cystamine dihydrochloride (450 mg, 2.0 mmol) and triethylamine (606 mg, 6.0 mmol) were added. mmol) at room temperature, stirring and dissolving, heating to 50°C for reflux for 3 hours, then cooling to room temperature, adding to a dialysis bag (molecular weight cut-off 800Da), dialyzing in ultrapure water for three days, and freeze-drying to obtain 84 mg of blue powdery solid. rate 87%.
[0051] The synthesized cystamined neoindocyanine green was characterized by 1H NMR and HPLC. like figure 2 As shown, compared with free neo indocyanine green, the characteristic peaks (a, b) of the methylene group in the cystamine group appeared in the H NMR spectrum of cystaminated neo indocyanine green, indicating that the cystaminated neo indocyanine green Successful ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com