Marker composition for colorectal cancer detection and application thereof
A technology of colorectal cancer and composition, applied in the field of marker composition for colorectal cancer detection, can solve problems such as side effects of treatment, excessive anxiety of the crowd, economic loss, etc., to improve sensitivity and specificity, and realize early screening and early diagnosis The effect of avoiding overdiagnosis and treatment of problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The embodiment of the present invention provides a marker composition for colorectal cancer detection, the marker composition is used for distinguishing benign and malignant intestinal polyps and detecting 57 plasma-free DNA methylation markers of colorectal cancer. at least one and the application. Specifically, it includes the following gene fragments with significant differential methylation in the plasma of colorectal cancer patients and intestinal polyps patients: chr13:46756082-46756083, chr16:29757360-29757361, chr16:29757375-29757376, chr1:211780424-211780425, chr16:29757350-29757351、 chr12:123707827-123707828、chr12:123707825-123707826、 chr1:211780438-211780439、chr20:55968287-55968288、 chr13:46756209-46756210、chr7:11208563-11208564、chr10:11207969-11207970、 chr13: 46756193-46756194、chr10:11207977-11207978、 chr5:115152492-115152493、chr5:115152479-115152480、 chr13:78493280-78493281、chr10:11207913-11207914、chr19:58095644-58095645、 chr6:393188-393189、chr16:86544859- ...
Embodiment 2
[0046] Example 2: Detection method of blood protein marker concentration and cfDNA methylation marker for colorectal cancer detection:
[0047] 1. The specific experimental steps of protein labeling detection are as follows:
[0048] 1. Sample processing: The serum should be separated within 24 hours after the venous blood collection. The hemolyzed and lipid blood samples should not be used for testing and must be resampled.
[0049] 2. Preparation before the test: first turn on the power of the instrument (MQ60 PLUS automatic chemiluminescence immunoassay analyzer), start initialization, and start the test 30 minutes after the instrument is turned on. Take out the separated serum samples. If the samples are frozen, they need to be thawed and equilibrated at room temperature for 30 minutes. At the same time, the kits and standards are taken out and equilibrated at room temperature for 30 minutes.
[0050] 3. Calibration: Add 500 μL of calibrator dilution solution to each of c...
Embodiment 3
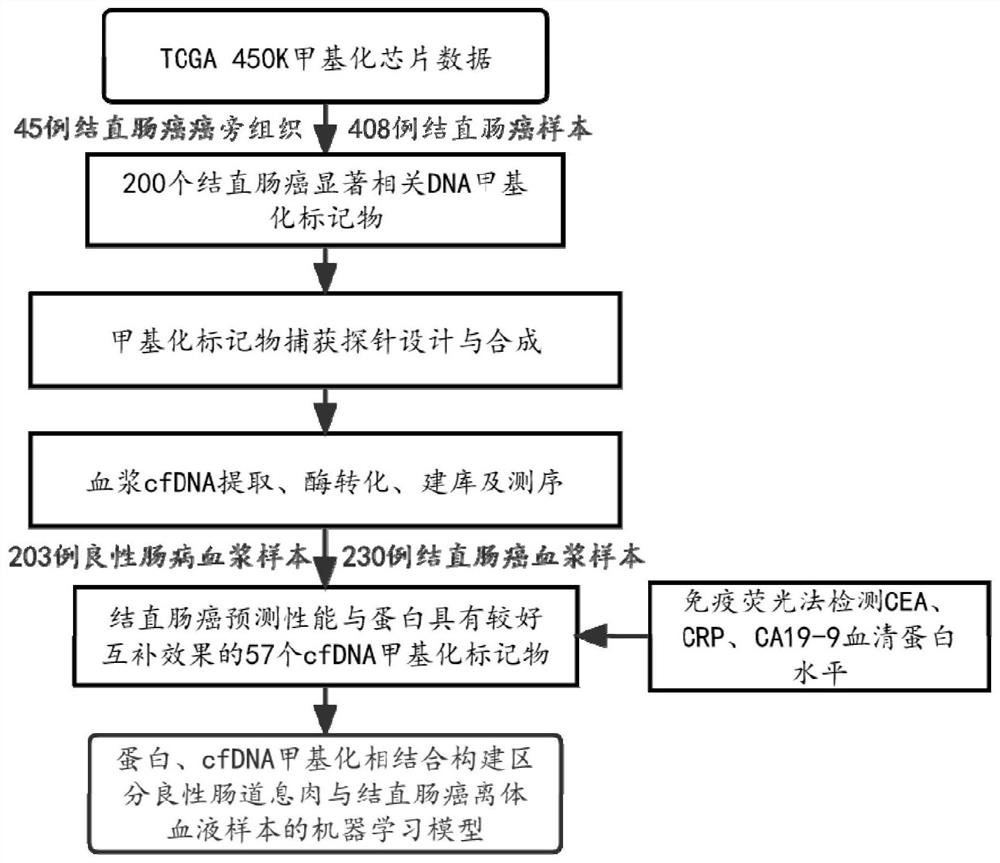
[0154] In this example, based on 45 colorectal cancer adjacent tissue samples in the TCGA public database, 408 colorectal cancer tissue samples were mined to find 200 DNA methylation markers that were significantly differentially methylated in colorectal cancer samples. Design and synthesis of capture probes. Further, this example uses plasma samples from 203 patients with benign intestinal polyps and plasma samples from 230 patients with colorectal cancer to extract cfDNA, build a library, and sequence. Finally, 57 cfDNA methylation markers whose predictive performance and protein markers (CRP, CEA, CA19-9) were well complemented were screened out by random forest model for distinguishing benign intestinal polyps and colorectal cancer patients. The whole analysis process is as figure 1 shown.
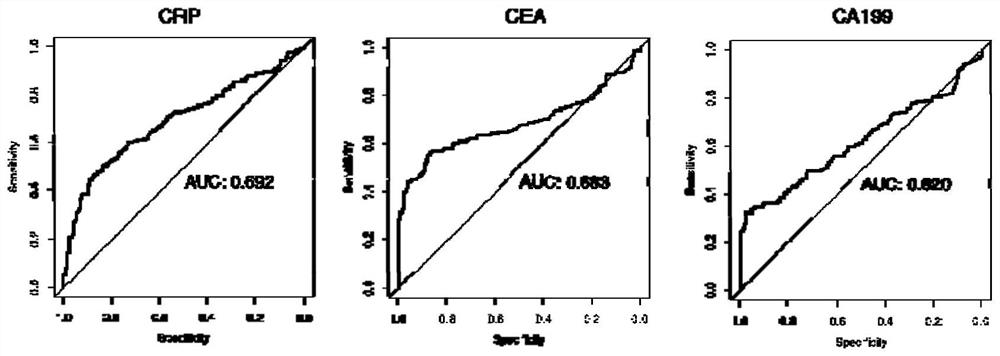
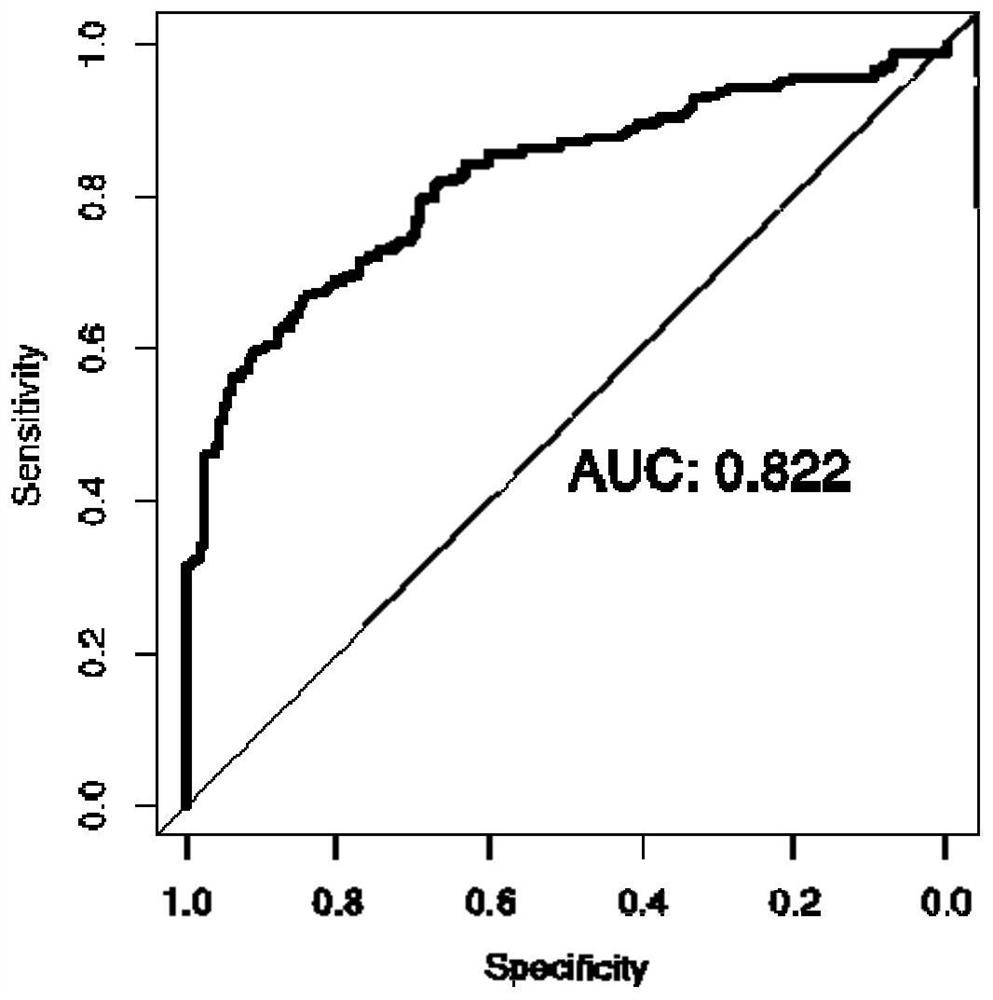
[0155] like figure 2 As shown, this example discloses a combination of protein markers for detecting colorectal cancer, the AUC value of CRP alone for predicting colorectal cancer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com