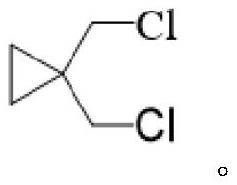
Montelukast sodium intermediate, preparation method thereof and preparation method of montelukast sodium intermediate
A technology for montelukast sodium and intermediates, applied in the field of medicinal chemistry, can solve the problems affecting the large-scale production of montelukast sodium intermediates, long synthetic routes, high production costs, etc., and achieve low production costs and simple post-processing , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
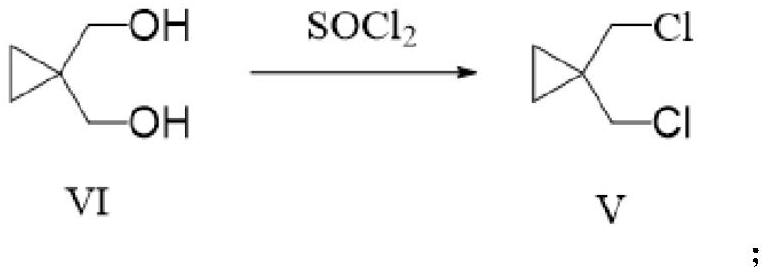
[0039] The preparation of embodiment 1 compound V
[0040]
[0041] Compound VI (30.6 g, 0.3 mol) was dissolved in 300 ml of dichloromethane. To this mixture was added SOCl 2 (166.6 g, 1.4 mol). The obtained mixture was heated to 40-80° C. for 8-12 hours, and then the obtained mixture was evaporated to dryness to obtain 38.04 g of compound V with a yield of 91.2% and a purity of 99.2%.
Embodiment 2
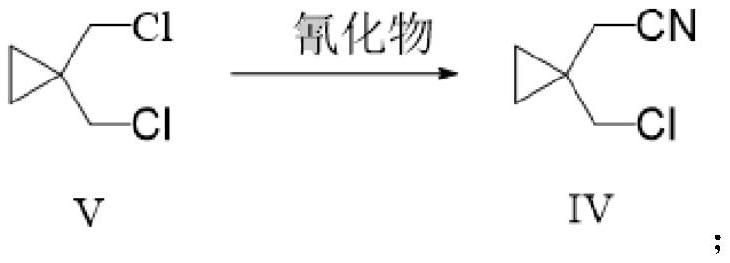
[0042] The preparation of embodiment 2 compound IV
[0043]
[0044] Under nitrogen protection, compound V (27.8 g, 0.2 mol) was added to 100 ml of N,N-dimethylformamide, followed by sodium cyanide (14.7 g, 0.3 mol). The reaction mixture was heated to 80°C and stirred for 2 hours. After the reaction was completed, the resulting mixture was cooled to 20-25°C. 100 mL of water and 100 mL of toluene were added to the reaction mixture, the layers were separated, the aqueous layer was back-extracted with 100 mL of toluene, the organic layers were combined, and concentrated to obtain 24.57 g of compound I with a yield of 94.8% and a purity of 99.5%.
Embodiment 3
[0046]
[0047] (1) Preparation of compound III
[0048] Compound IV (25.9 g, 0.2 mol), thiourea (16 g, 0.21 mol) and 100 ml of acetone were added to the reaction flask, and the mixture was stirred and heated to reflux for 10 hours. The reaction mixture was then cooled to below 0°C, stirred for at least 1 hour, and filtered to obtain a filter cake, which was washed with 2 x 50 ml of acetone. Then, 100 ml of acetone was added to the container containing the filter cake and slurried for 5 hours. The mixture was filtered and washed with 2×25 ml of acetone, and after vacuum drying, 40.44 g of compound III was obtained in a yield of 98.3% and a purity of 99.5%.
[0049] (2) Preparation of Compound I
[0050] Under nitrogen protection, compound III (20.6 g, 0.1 mol) and 60 ml of 30% sodium hydroxide solution (containing 0.6 mol of sodium hydroxide) were added to the reaction flask. The mixture was stirred and heated to reflux for about 14 hours. After cooling to room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com