Method for continuously synthesizing 1, 1, 1, 3, 3-pentachloropropane
A synthesis method and technology of pentachloropropane, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, preparation of halogenated hydrocarbons, etc., can solve low production efficiency, difficulty in large-scale production, and environmental damage And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
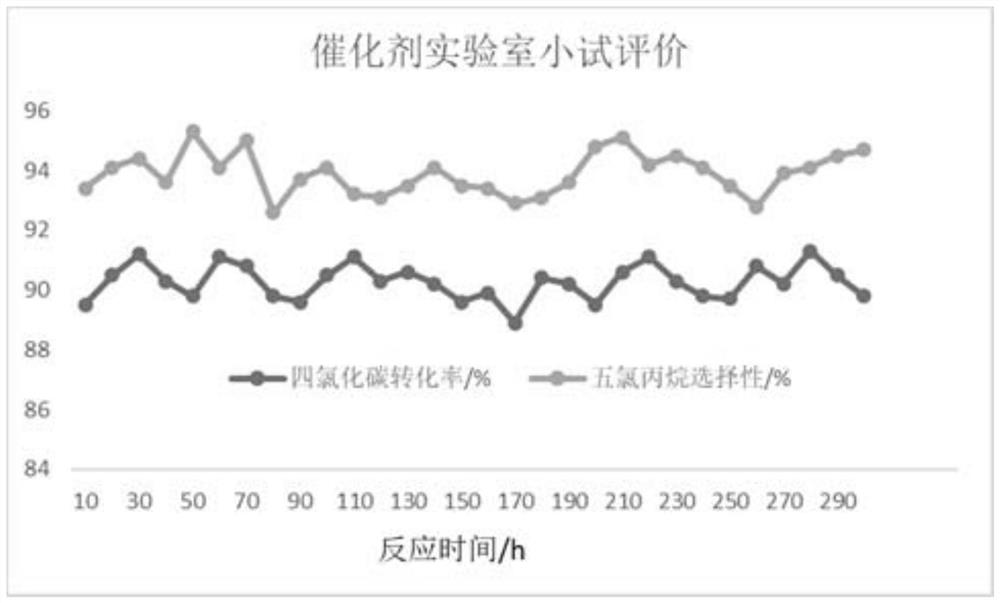
Image
Examples
Embodiment 1
[0049] Weigh 500g of carbon tetrachloride raw materials, ethyl phosphite and vinyl chloride, and mix them evenly. The amount of ethyl phosphite is 2%, and the molar ratio of vinyl chloride to carbon tetrachloride is 1:2. The reactants are preheated to 90°C. Begin to enter the tubular reactor, the volumetric space velocity of vinyl chloride is 50h -1 . The reaction tube is a quartz tube with an inner diameter of 20 mm, and is filled with fine iron balls according to 70% of the volume of the reactor, the reaction temperature is controlled to 90 ° C, the reaction pressure is 0.4 MPa, and the reaction time is 40 minutes. 1,1,3,3-pentachloropropane, and the separated carbon tetrachloride is refluxed into the reaction feed liquid to continue the reaction. The conversion rate of vinyl chloride is 80.5%, and the selectivity of 1,1,1,3,3-pentachloropropane is 96.5%.
Embodiment 2
[0051] Weigh 500g of carbon tetrachloride raw materials, butyl phosphate and vinyl chloride are fully mixed, the amount of butyl phosphate is 5%, and the molar ratio of vinyl chloride and carbon tetrachloride is 1:3. The reactants are preheated to 60 ° C and begin to enter Tubular reactor with vinyl chloride volume space velocity of 120h -1 . The reaction tube adopts a quartz tube with an inner diameter of 20mm, and is filled with iron filings according to 70% of the volume of the reactor. The reaction temperature is controlled to 60°C, the reaction pressure is 0.7MPa, and the reaction time is 60 minutes. The crude product is collected by cold trap, and the crude product is analyzed by gas chromatography 1,1 , 1,3,3-pentachloropropane, and the separated carbon tetrachloride is refluxed to the reaction feed liquid to continue the reaction. The conversion rate of vinyl chloride is 65.5%, and the selectivity of 1,1,1,3,3-pentachloropropane is 97.5%.
Embodiment 3
[0053] Weigh 500g of carbon tetrachloride raw material, butyl phosphite and vinyl chloride are fully mixed, the amount of butyl phosphite is 3%, the molar ratio of vinyl chloride to carbon tetrachloride is 1:1, and the reactant is preheated to 120 ° C Begin to enter the tubular reactor, the volumetric space velocity of vinyl chloride is 80h -1 . The reaction tube adopts a quartz tube with an inner diameter of 20 mm, and is filled with Pall rings according to 70% of the volume of the reactor, and the reaction temperature is controlled at 120 ° C, the reaction pressure is 0.5 MPa, and the reaction time is 30 minutes. 1,1,3,3-pentachloropropane, and the separated carbon tetrachloride is refluxed into the reaction feed liquid to continue the reaction. The conversion rate of carbon tetrachloride is 85.5%, and the selectivity of 1,1,1,3,3-pentachloropropane is 95.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com