Application of small molecule compound in preparation of human La protein inhibitor
A technology of protein inhibitors and compounds, applied in the field of pharmaceuticals, can solve problems such as shortening, accelerated cell proliferation, and out of control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Computer high-throughput virtual screening docking screening of La protein inhibitor candidate compounds
[0035] use The software Maestro module is used for the docking analysis of La proteins with compounds in the 1.5 million ChemDiv compound library. The 2D ligand structures of the compound molecules were obtained from the ChemDiv compound library. The receptor structure was retrieved from the available crystal structures of the La protein and utilized Software's Protein Preparation Wizard module to build. The receptors were constructed using all chains of the protein structure, and water molecules more than 5 Angstroms away from the protein position were removed. Then, the potential allosteric sites of the La protein were predicted with AlloSitePro. Then, the prepared ligand was flexibly connected to the predicted binding site of the receptor with the default parameter Glide (XP mode). Finally, several docking poses of the molecule were obtained, and ...
Embodiment 2
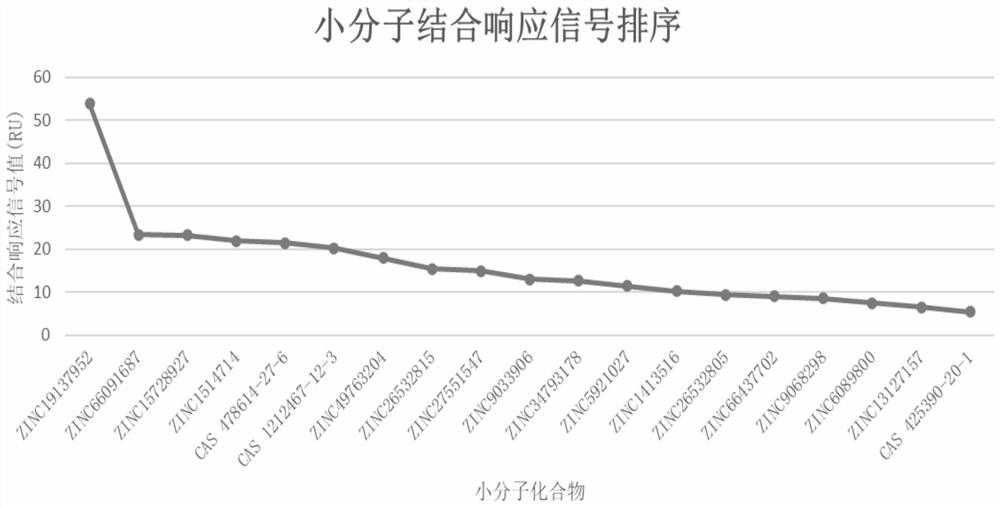
[0041] Example 2 SPR single-concentration primary screening to determine the affinity of human La protein and candidate compounds
[0042] 1. Expression and purification of recombinant human La protein in E. coli system
[0043] The full-length amino acid sequence of human La protein (containing 408 amino acids) was obtained from Pubmed protein, and the following sequence was added to the C-terminus of the obtained human La protein sequence: AGCCGGGGAGGCCAAAGCTTATCCACCCCGAGTGTAGATCTCGGTGGTCGCCGTATCATTGGTCTGAACGACATCTTCGAGGCTCAGAAAATCGAATGGCACGAACATCATCACCACCATCACTCT. Using MaxCodon TM Optimization Program (V13) software optimizes the above amino acid sequence, adopts whole gene synthesis and inserts the Lupus La gene into the expression vector pET30a through restriction enzyme sites NdeI and HindIII, and confirms the final expression vector by enzyme digestion and sequencing The accuracy was finally transferred to Top10 clone strain and BL21(DE3) expression strain respective...
Embodiment 3
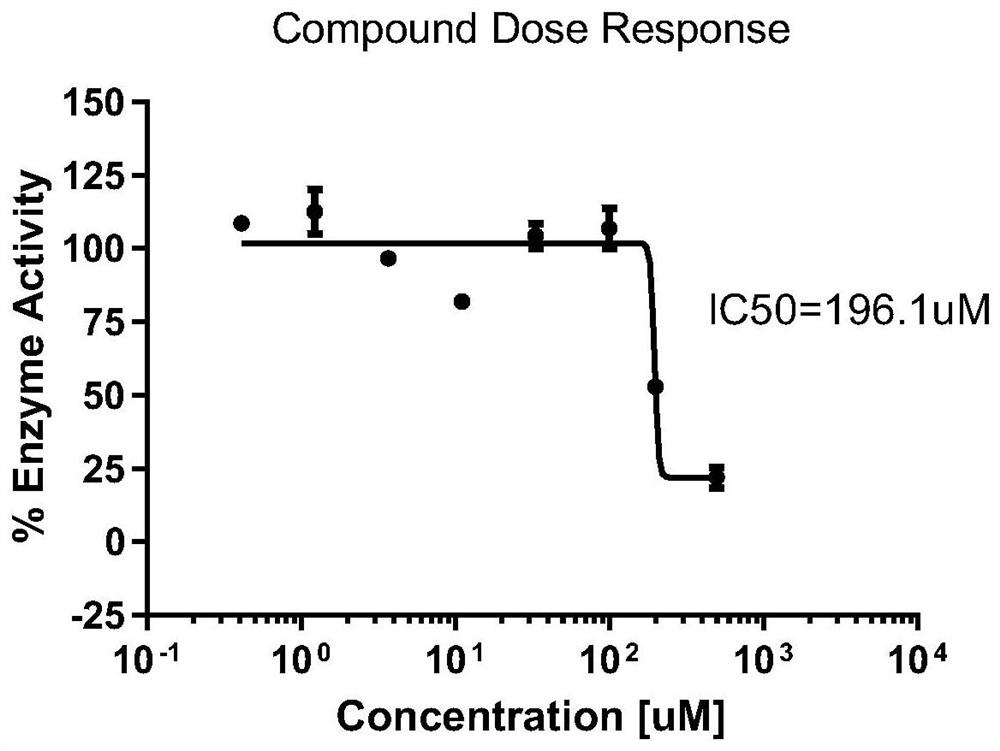
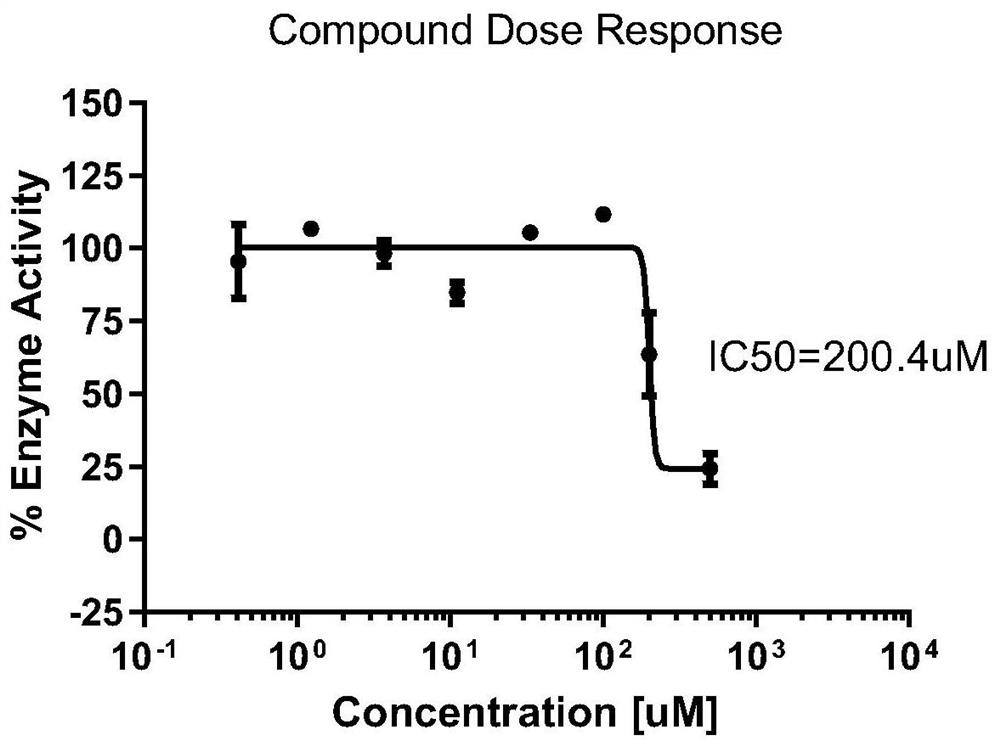
[0053] Example 3 Immunofluorescence polarization competition assay detects the in vitro inhibitory effect of compounds on La protein
[0054] 1. Construction of a fluorescence polarization detection system for the binding efficiency of human La protein and RNA probes
[0055] The synthetic scheme of recombinant human La protein is shown in Example 2. According to the binding site of La protein and RNA in the literature, 3 short-chain RNA probes (see Table 3) are designed. The probes are labeled with FAM fluorescence at the 5' end. Synthesized by Industrial Bioengineering (Shanghai) Co., Ltd.
[0056] table 3
[0057]
[0058] Table 3 RNA fluorescent probes used in immunofluorescence polarization competition experiments
[0059] Prepare assay buffer on ice: 50 mM Tris-HCl PH=8, 50 mM NaCl, 5 mM MgCl2, 2 mM DTT, 0.1 mM EDTA (prepared and used now). Use the prepared 1X assay buffer to dilute 10-fold in 100% DMSO to prepare a 10X working solution. The three RNA probes and r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com