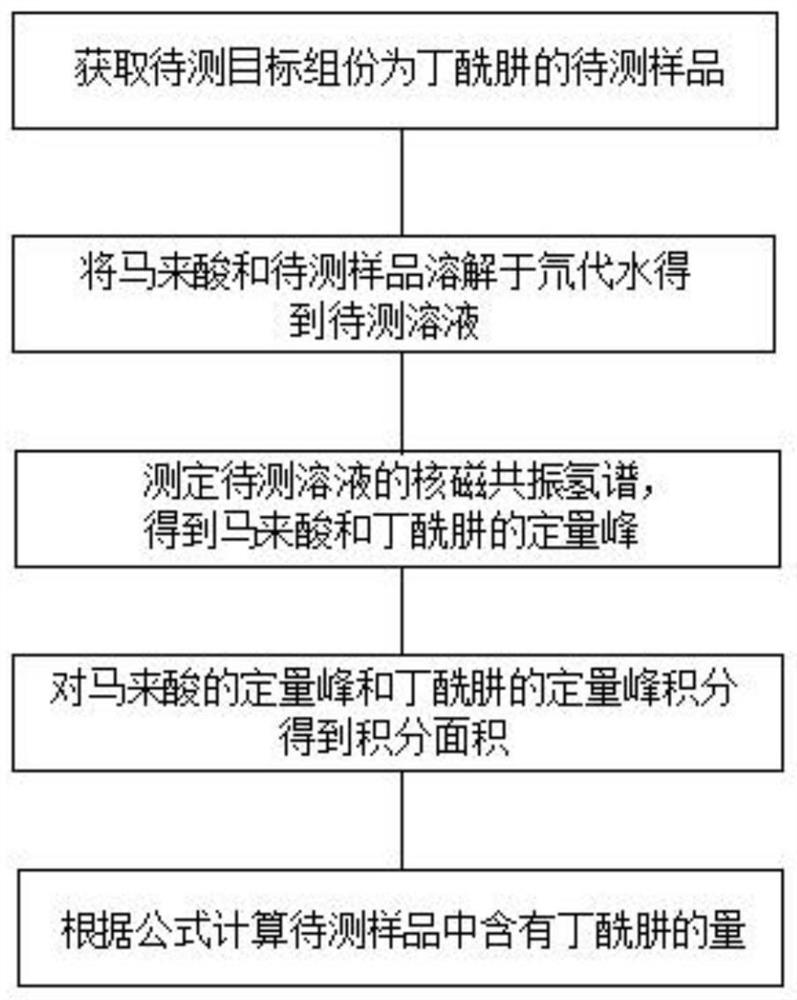
Method for quantitatively analyzing content of daminozide through nuclear magnetic resonance hydrogen spectrum
It is a technology of hydrogen nuclear magnetic resonance spectroscopy and quantitative analysis, which is applied in the analysis by nuclear magnetic resonance, material analysis by resonance, and material analysis. The effect of saving test efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
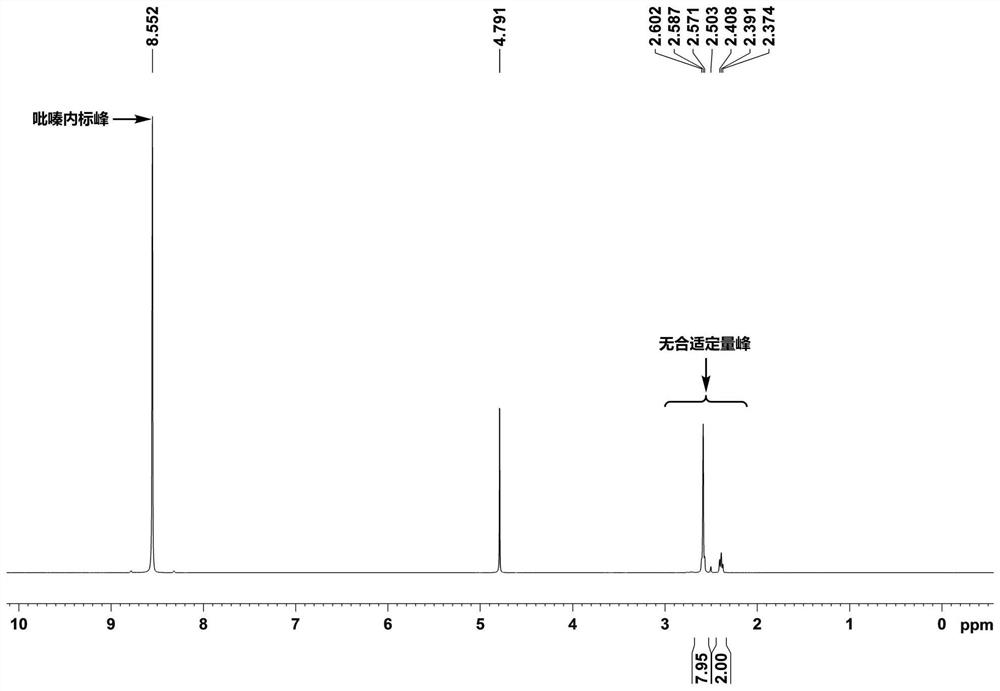
[0039] Butyryzide residues in peanuts were analyzed by 1H NMR spectroscopy. The specific steps are as follows:
[0040] Accurately weigh about 1.0 g (accurate to 0.0001 g) of the crushed and mixed sample into a 10 ml centrifuge tube, add 6 ml of heavy water, shake for 30 minutes and then centrifuge for 15 minutes (3000 r / min). Pipette 0.6ml of the supernatant into a 0.5mm nuclear magnetic tube for on-machine testing, and test and calculate the content of butyryl hydrazide according to the steps in the technical solution
[0041] Table 1 Test of butyryzide residues in peanuts
[0042]
Embodiment 2
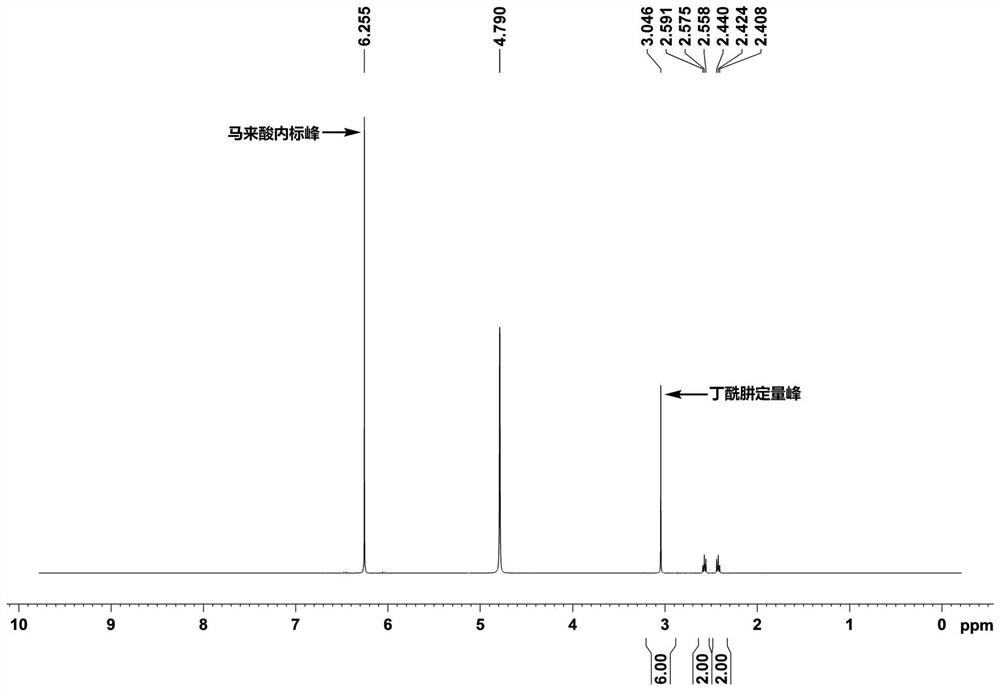
[0044] The purity of the butyryzide raw material was analyzed by hydrogen nuclear magnetic resonance spectroscopy, and the specific steps were as follows:
[0045]Weigh 50mg of butyryzide API, transfer it into a 2mL disposable PE centrifuge tube, add 1.5mL of standard solution, ultrasonicate for 5min, the solution is clear and transparent, add 0.6mL to the NMR tube, and test on the machine. Calculate the actual content of butyric hydrazide according to the following formula, and calculate the purity of the raw material according to the actual content of butyric hydrazide.
[0046]
[0047] Table 2 butyryzide raw material drug purity analysis
[0048]
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com