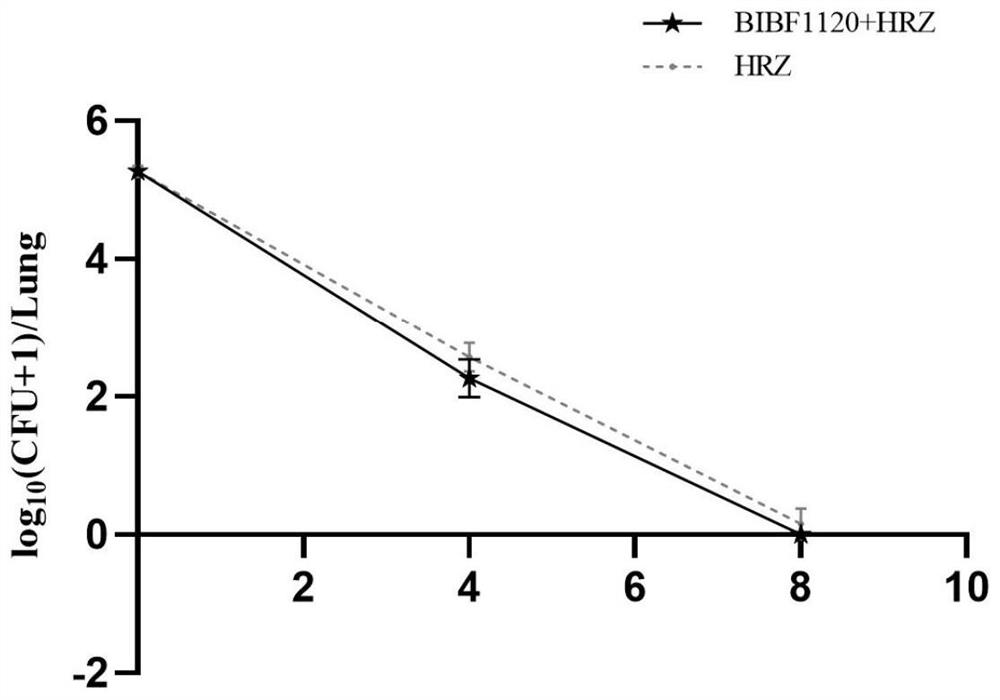
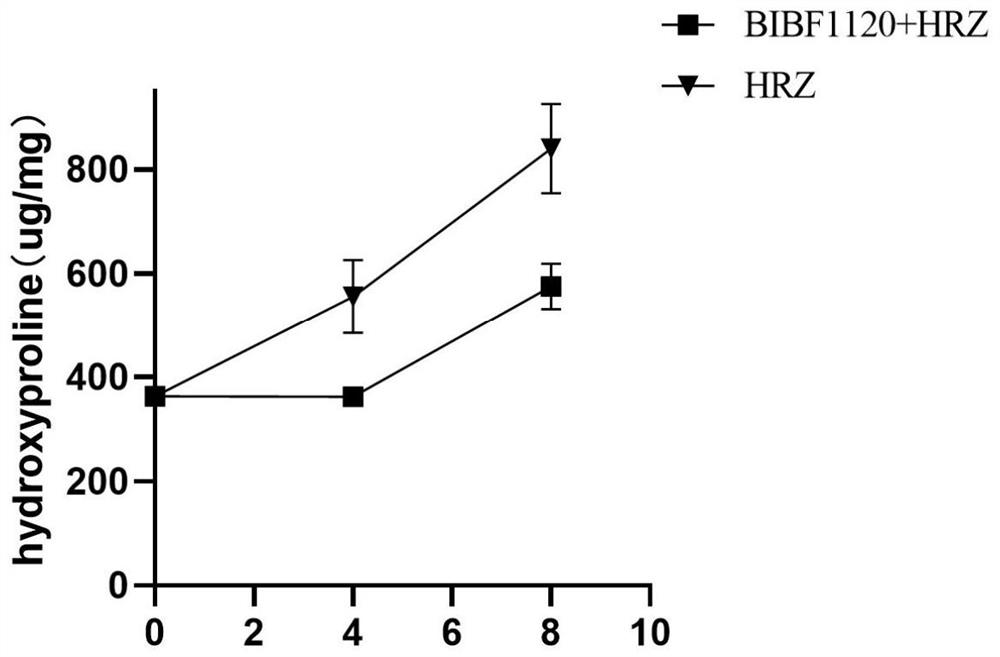
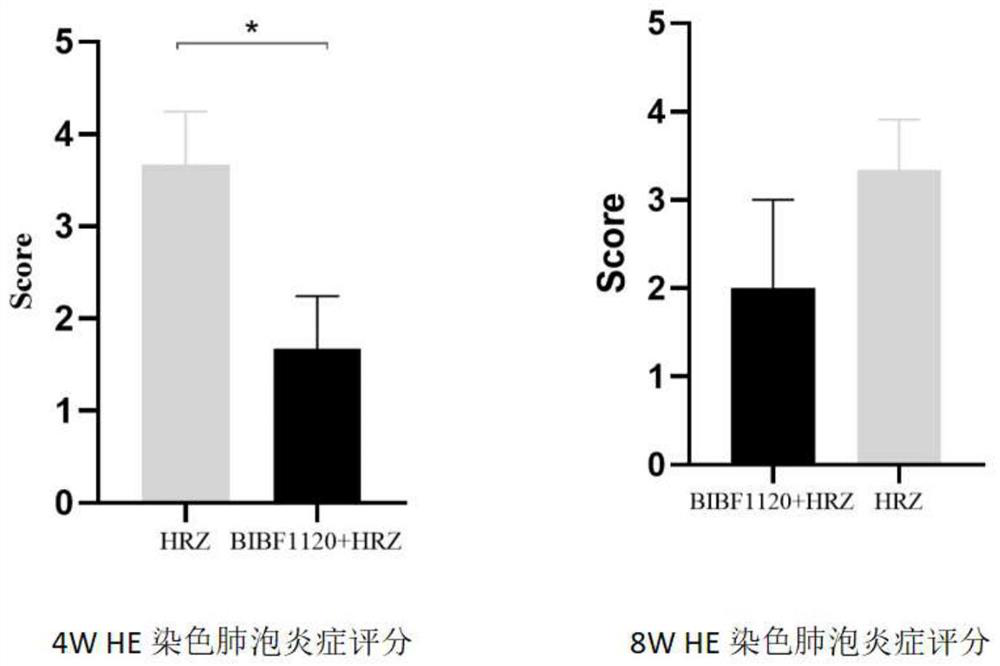
Application of medicine nintedanib for resisting idiopathic pulmonary fibrosis in tuberculosis treatment
A technology of nintedanib and nintedanib ethyl sulfonate, applied in the field of new drug use, can solve problems such as nintedanib that have not been seen, and achieve the effects of low recurrence rate and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0036] Experimental Example 1 In vitro activity of nintedanib against Mycobacterium tuberculosis
[0037] The H37Rv strain was cultivated to the logarithmic growth phase, and the bacterial suspension was diluted to 1 × 10 in 7H9 liquid medium. 6 CFU / ml for use. As shown in the figure below: Rows A-F are positive / negative control wells, INH, RFP, PFD, SC1011, BIBF1120. Take a 96-well plate and sequentially dilute each drug-containing well. After culturing at 37°C for 7 days, 20 μl of alamar blue and 12.5 μl of 20% Tween-80 were added to each well, and the culture was continued at 37°C for 24 h, and the color of each well was recorded. Indicates the lowest drug concentration that changes from blue to red. Repeat the test 3 times. The results showed that nintedanib had bacteriostatic activity, and the MIC value for H37Rv standard strain was 24.567ug / ml.
experiment example 2
[0038] Experimental example 2: The effect of nintedanib on the anti-tuberculosis drug rifampicin
[0039]The minimum drug concentration (MIC90) when the drug inhibited 90% of the growth of Mycobacterium tuberculosis was determined by the micro-broth dilution method, and then based on the MIC value of each drug alone, BIBF1120 and RFP were diluted by double dilution method as 6 concentrations, consisting of 2×MIC-1 / 16×MIC. Add 50 μl of 2MIC-1 / 16MIC A solution (BIBF1120) to the second column of the 96-well plate, respectively, and add 50 μl of 2×MIC-1 / 16×MIC B solution (RFP) to the first 7 wells of rows 2-7. Add 50 μl of 7H9 to each single-drug MIC assay well, and finally add 100 μl of the diluted bacterial suspension to each well of the 96-well plate, and set positive control wells (100 μl diluted bacterial solution + 100 μl 7H9) and negative control wells (200 μl 7H9 ). The MIC of a single drug in a drug combination was determined by observing the color change in the 96-well...
experiment example 3
[0040] Experimental example 3: bactericidal activity of nintedanib at different concentrations in macrophages
[0041] The anti-tuberculosis activity of BIBF1120 alone and the combination of BIBF1120+RFP was evaluated by the intracellular bactericidal activity of macrophages. The specific operation method is as follows: collect J774A.1 macrophages into a 50ml centrifuge tube, take 100ul of macrophages and 900ul of cell culture medium, and add them to a 1.5ml sterile centrifuge tube for 10-fold dilution. Counted under the microscope. Dilute macrophages to 4*10 5 pcs / ml, gently spread in a 48-well transparent microplate, 1ml per well. After plating, put it into a 37°C, 5% CO2 incubator for 24h. MOI=5:1 and 2*106CFU / MLH37Rv Co-culture with macrophages. After 4 h, the cells were washed twice with sterile PBS to remove extracellular Mycobacterium tuberculosis, and new RPMI cell culture medium was added. Put into a 37°C 5% CO2 incubator for 72h. After 3 days, discard the RPMI i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com