Preparation method of cattle beta-defensin 5 mature peptide, its recombinant bacteria and application thereof
A defensin, mature peptide technology, applied in the application of bovine beta-defensin 5 mature peptide in animal husbandry production and drug treatment, the recombinant bacteria expressing bovine beta-defensin 5 mature peptide, bovine beta-defensin 5. The field of preparation of mature peptides can solve the problems of high cost, inability to meet needs, low content of natural defensins, etc., and achieve the effect of low production cost, high production efficiency and maintaining natural structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of bovine neutrophil β-defensin 5 mature peptide gene
[0040] 1. Design the primers for synthetic bovine neutrophil β-defensin 5 mature peptide (mBNBD5) gene
[0041] According to the nucleotide sequence of the CDS region of BNBD5 published on GenBank (sequence number: AJ278799.1) and the restriction site of the eukaryotic expression vector pPIC9K, the upstream primer was designed at the beginning of the gene, and the EcoRI restriction site ( Underlined), 3 protective bases GCC are added before the EcoRI restriction site; the downstream primer is designed at the end of the gene, and a Not I restriction site (underlined) is added. At the same time, in order to facilitate future protein purification, downstream A 6-His tag (underlined under the wavy line) is added before the C-terminal stop codon of the primer, and 3 protective bases GCC are added before the NotⅠ restriction site, which was synthesized by Genewiz Biotechnology Co., Ltd. They are:
[0042...
Embodiment 2
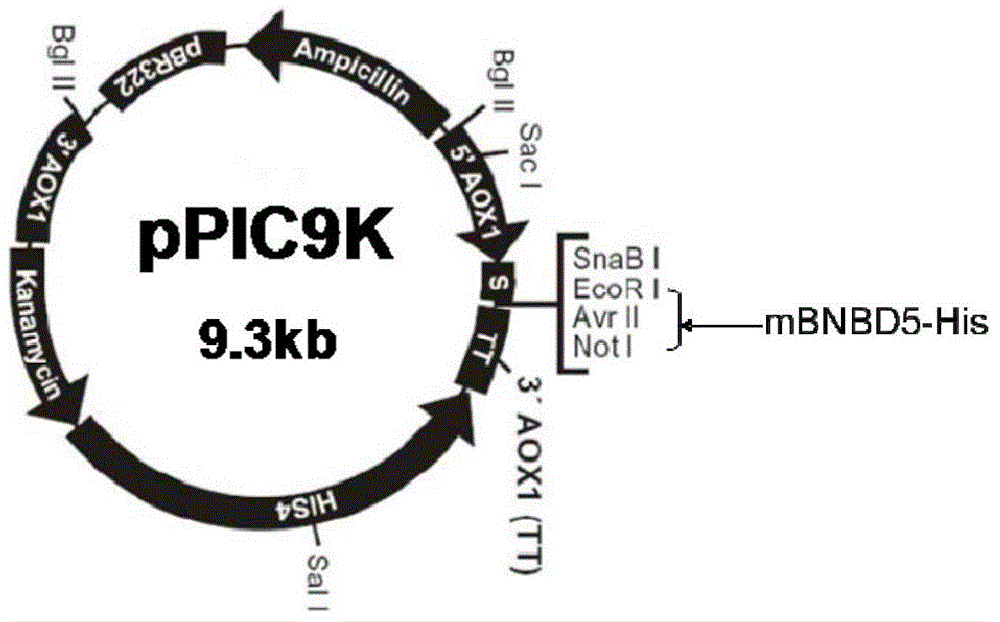
[0048] Example 2 Construction of recombinant plasmid pPIC9K-mBNBD5
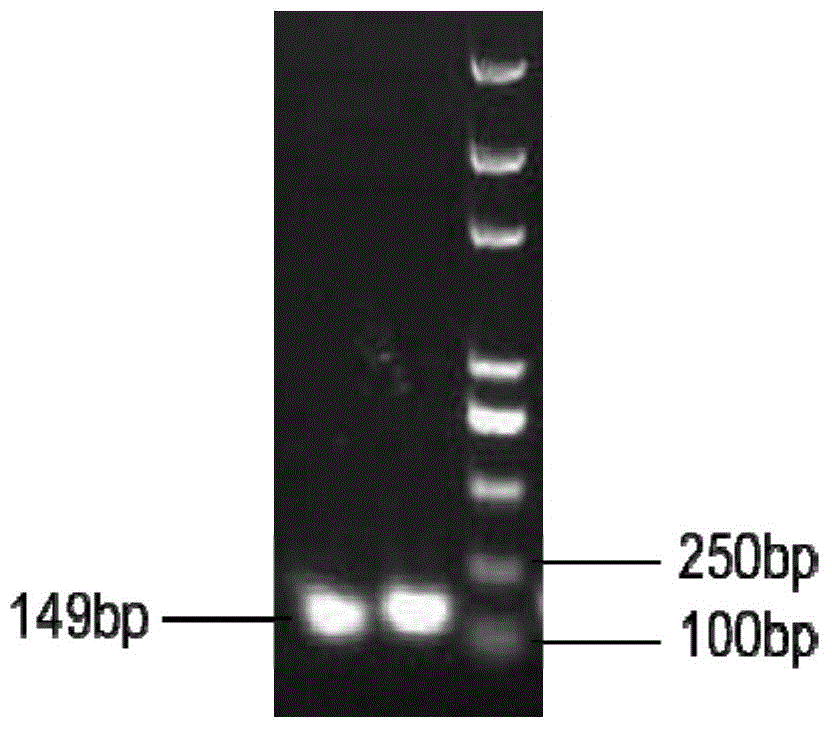
[0049] The obtained PCR product of bovine neutrophil β-defensin 5 mature peptide (mBNBD5) was purified and recovered with a rapid agarose gel DNA recovery kit. The recovered product was double digested with endonucleases EcoRI and Not I After treatment, a band with a size of about 160 bp was recovered by the gel; the eukaryotic expression vector pPIC9K was subjected to double enzyme digestion treatment with the same method, and a DNA fragment with a size of about 9 kb was recovered by the gel. Neutrophil β-defensin 5 mature peptide (mBNBD5) gene fragment and 2μL of the eukaryotic expression vector pPIC9K recovered by double enzyme digestion, adding 2μL of 5×T4DNA ligation buffer, 1μL of T4DNA ligase, supplemented with autoclaving ddH 2 O to 10μL, ligate at 16℃ for 16h, transform the ligation product into E. coli TOP10 competent cells, and spread 200μL on LB / Amp + Incubate on solid medium at 37°C for 14-16h to co...
Embodiment 3
[0051] Example 3 Preparation of Pichia pastoris genetic engineering strain
[0052] 1. Preparation and linearization of recombinant plasmid
[0053] Select the TOP10 positive bacteria transformed into the recombinant plasmid pPIC9K-mBNBD5 and inoculate 100 mL of LB / Amp + In a liquid medium, incubate at 37°C for 12-14h, centrifuge the bacterial solution at 4°C, 5000rpm for 30min, collect the precipitated bacteria, and then use endotoxin-free plasmid DNA extraction kit to extract the plasmid, and at the same time extract the empty plasmid pPIC9K Plasmid DNA was used as a blank control. The extracted recombinant plasmid pPIC9K-mBNBD5 and the empty plasmid pPIC9K were linearized with endonuclease SacI. In order to facilitate electrotransformation, the linearized recombinant plasmid pPIC9K-mBNBD5 and the empty plasmid pPIC9K were purified by phenol / chloroform extraction, added with absolute ethanol (pre-cooled at -20°C) to precipitate the DNA, and after the precipitate was naturally dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com