Method for synthesizing heptasaccharide of lentinan core fragment beta-(1-> 6) branched chain beta-(1-> 3) main chain
A technology of core fragments and lentinan, applied in chemical instruments and methods, drug combinations, sugar derivatives, etc., can solve the problem of normal cell killing, etc., and achieve the effect of extremely simple route, high yield and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
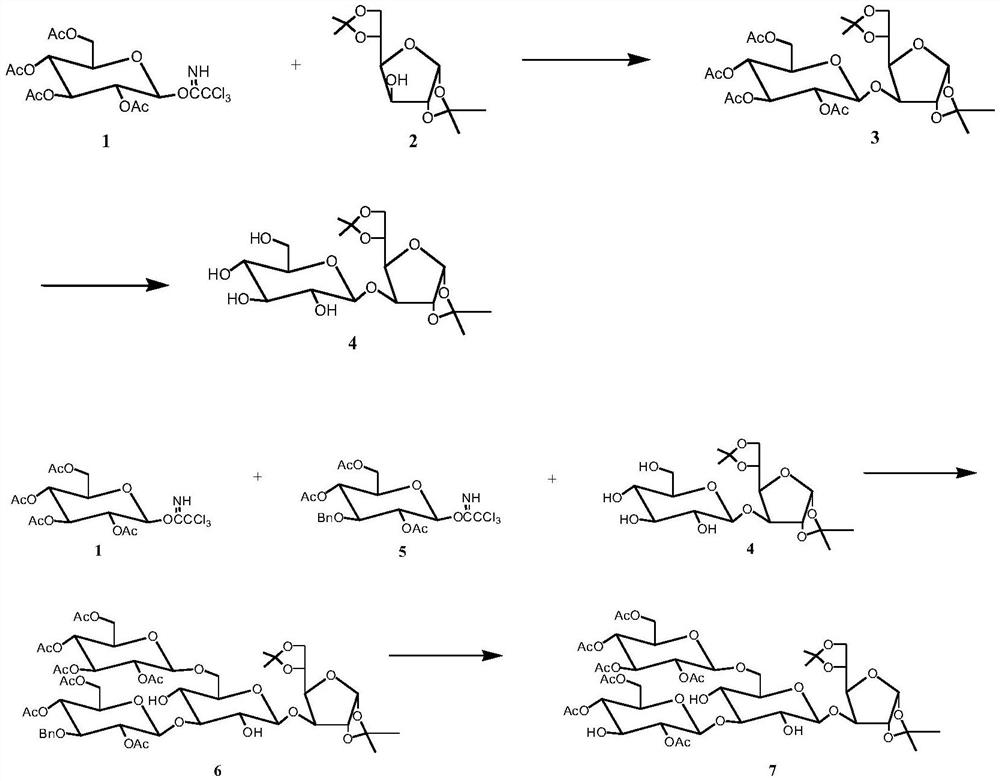
[0039] Preparation of Tetrasaccharide Acceptor 7 and Trisaccharide Donor 9
[0040] (1) 2,3,4,6-Tetra-O-acetylglucose trichloroacetimidate 1 (2.96 g, 6 mmol) was dissolved in 10 mL of dichloromethane to give solution A, 1,2- 5,6-Di-O-isopropylidene glucose 2 (1.56 g, 6 mmol) was dissolved in 10 mL of dichloromethane to give solution B, A and B were mixed to give solution C, to C was added TMSOTf (28 μL, 0.25 mmol), stirred at 25°C, and after 2-4 hours of reaction, thin layer chromatography analysis indicated that the reaction was complete. The solvent was evaporated under reduced pressure, and the disaccharide 3 was obtained by coupling. Disaccharide 3 (3.54 g, 5.4 mmol) was dissolved in 20 ml of alkanolamine-dichloromethane, stirred at 25° C. After 8-10 hours of reaction, thin-layer chromatography analysis indicated that the reaction was completed, and the solvent was evaporated under reduced pressure to obtain Disaccharide 4 in 94.1% yield.
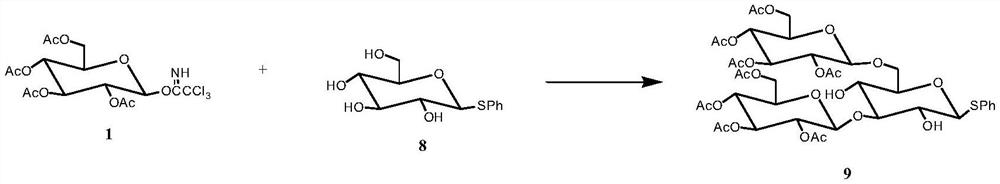
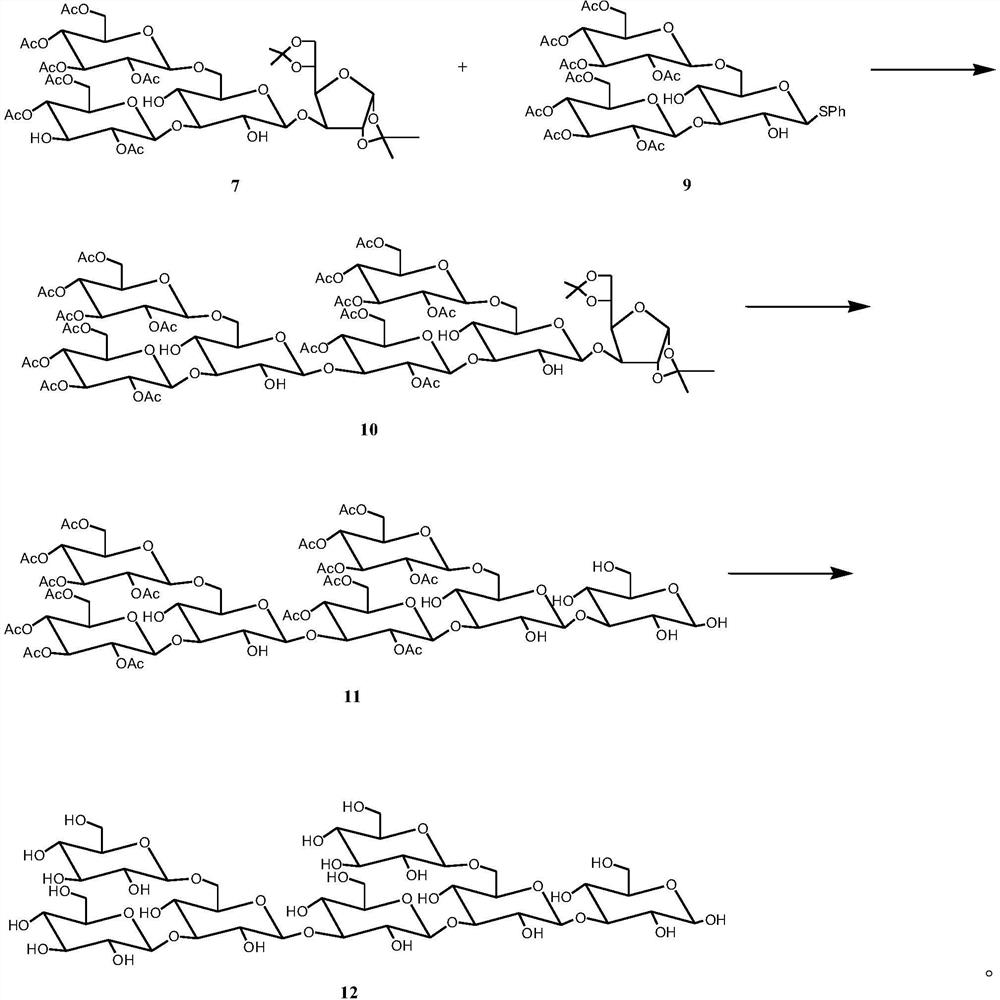
[0041] (2) 2,3,4,6-Tetra-O-ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com