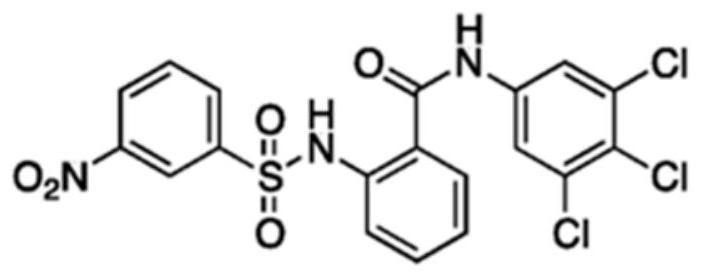
Preparation method of IMB16-4 liposome nanoparticles and medicine
A nanoparticle and liposome technology, which is applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as poor patient compliance, low bioavailability, and inability to deliver drugs , to reduce toxic and side effects, improve solubility and absorption, and improve curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
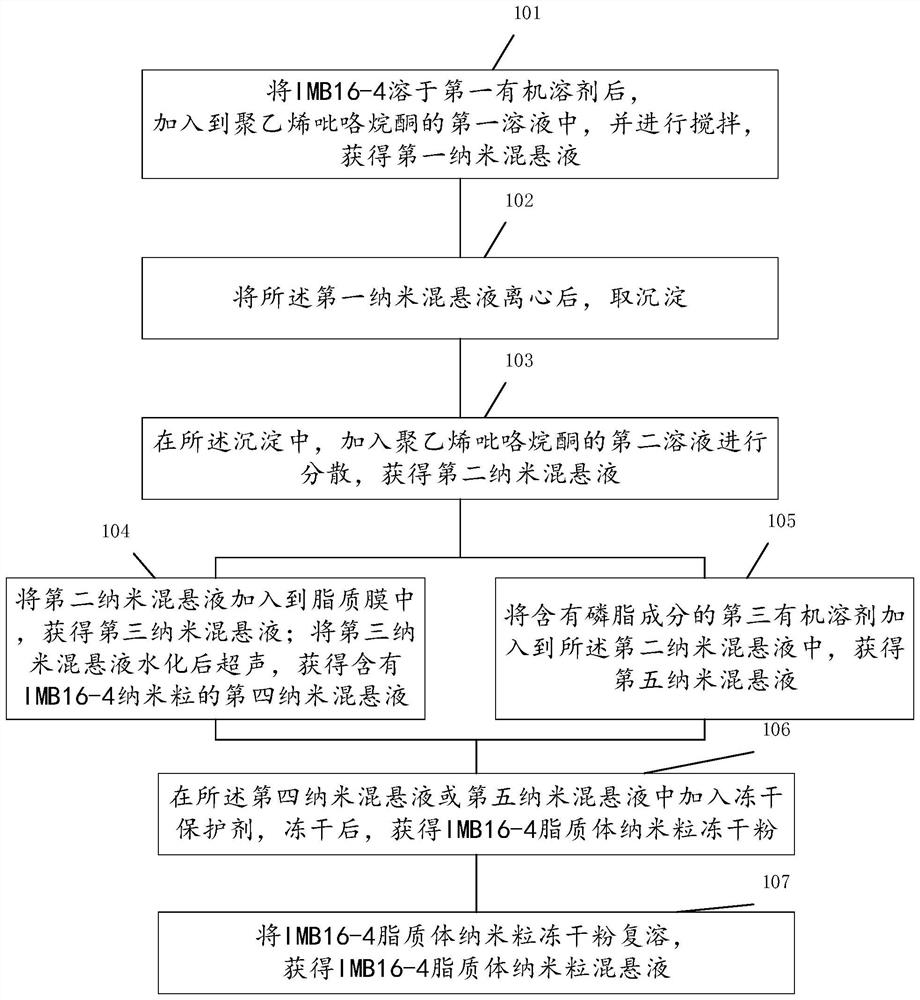
[0040] A preparation method of IMB16-4 liposome nanoparticle, such as figure 2 As shown, the preparation method includes:
[0041] Step 101: After dissolving IMB16-4 in the first organic solvent, add it to the first solution of polyvinylpyrrolidone (eg PVP K30), and stir to obtain the first nanosuspension. The first organic solvent includes, but is not limited to, dimethylformamide (DMF) or dimethylformamide (DMSO). Polyvinylpyrrolidone was used as a stabilizer to attach to the surface of IMB16-4 nanoparticles to stabilize them.
[0042] Step 102: After centrifuging the first nanosuspension, take a precipitate.
[0043]Step 103 : in the precipitation, add a second solution of polyvinylpyrrolidone for dispersion to obtain a second nanosuspension containing IMB16-4 nanoparticles, and perform step 104 or 105 . Wherein, the first solution or the second solution is a 0.1-0.5% w / v polyvinylpyrrolidone (eg PVP K30) aqueous solution. PVP K17, K12, etc. can also be used for polyvi...
Embodiment 1
[0058] Screening of the second organic solvent:
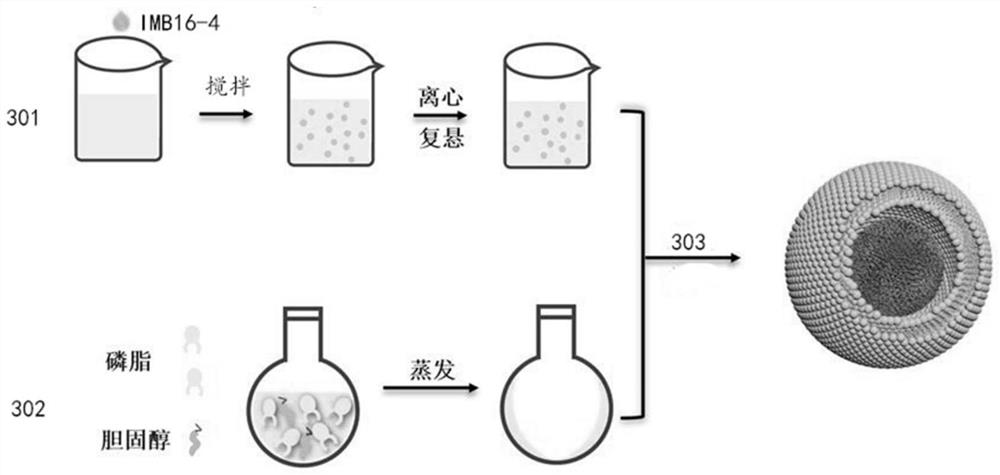
[0059] image 3 The preparation process of liposome nanoparticles is shown:
[0060] Step 301: Preparation of IMB16-4 nanoparticles: Dissolve 10 mg of IMB16-4 in 1 mL of DMF, and after complete dissolution, add dropwise to 10 mL of 0.1% w / v PVP K30 solution while magnetically stirring to obtain the first nano-mixture. suspension. Centrifuge the first nanosuspension, discard the supernatant, and take the pellet. 10 mL of 0.1% w / v PVP K30 was added to the precipitate for dispersion, and a nanosuspension with pale blue opalescence, namely the second nanosuspension, was obtained.
[0061] Step 302: Preparation of lipid film: Dissolve 15 mg of egg yolk lecithin and 3 mg of cholesterol in 6 mL of a second organic solvent, remove the second organic solvent by rotary evaporation, and obtain a lipid film at the bottom of the container.
[0062] Step 303: Preparation of IMB16-4 liposome nanoparticles: add the second nanosuspension (I...
Embodiment 2
[0070] Screening of phospholipid components:
[0071] Step 401: Preparation of IMB16-4 nanoparticles: Dissolve 10 mg of IMB16-4 in 1 mL of DMF, and after complete dissolution, add dropwise to 10 mL of 0.1% w / v PVP K30 solution while magnetically stirring to obtain the first nano-mixture. suspension. Centrifuge the first nanosuspension, discard the supernatant, and take the pellet. 10 mL of 0.1% w / v PVP K30 was added to the precipitate for dispersion, and a nanosuspension with pale blue opalescence, namely the second nanosuspension, was obtained.
[0072] Step 402: Preparation of lipid film: Dissolve phospholipid, DOTAP and cholesterol in 6 mL of n-butanol, remove the organic solvent by rotary evaporation, and obtain a lipid film at the bottom of the container.
[0073] Step 403: Preparation of IMB16-4 liposome nanoparticles: add the second nanosuspension (IMB16-4 nanoparticle concentration: 0.5 mg / mL) obtained in step 401 to the lipid film in step 402, at room temperature H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com