Application of rotavirus non-structural protein 4 in improving immune function of recombinant rotavirus subunit vaccine
A subunit vaccine, non-structural protein technology, applied in the field of vaccine preparation, to avoid the risk of intussusception, improve protection, and enhance the effects of immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
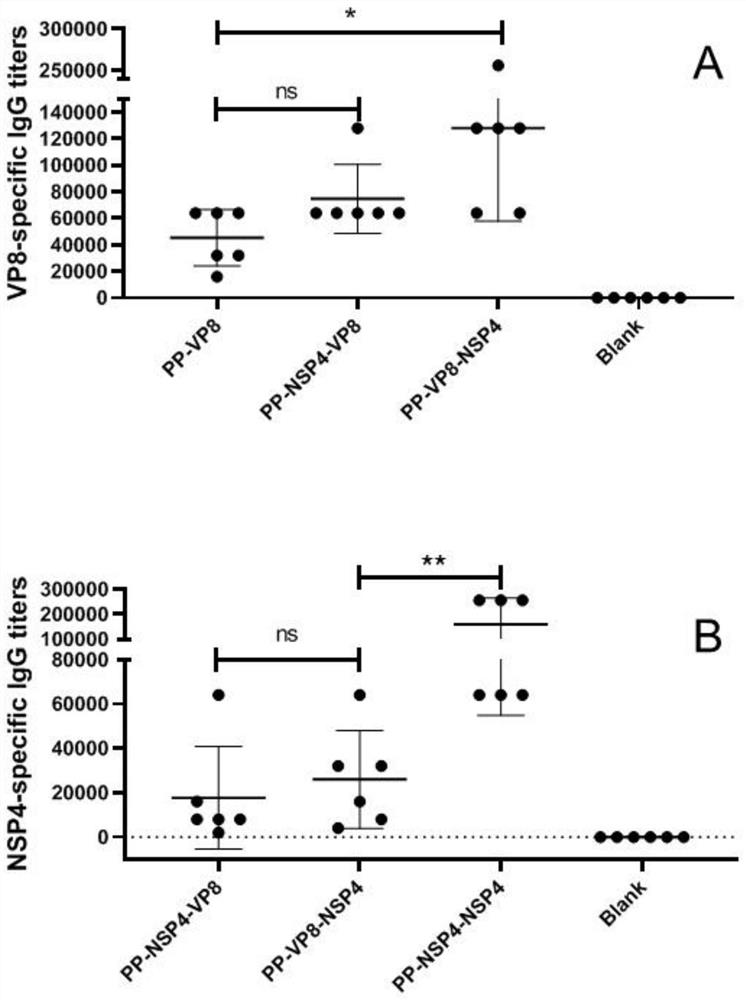
[0026] Four antigens were designed below. The expression vectors for the four antigens were the loop2 loop and / or the loop3 loop surface loop of the protruding domain of Norovirus VA387 (genome II, cluster 4 [GII.4]).
[0027] The partial nucleotide sequence of the Norovirus VA387 expression vector containing the surface loops of loop2 and loop3 is shown in SEQ ID NO: 1, and the amino acid sequence is shown in SEQ ID NO: 2.
[0028] (1) PP-VP8: The VP8 antigen gene is inserted into the surface loop of loop2 for expression, and the insertion site is between T369-D372 of the amino acid sequence shown in SEQ ID NO: 2.
[0029] (2) PP-NSP4-VP8 (tandem expression): both NSP4 gene and VP8 antigen gene are inserted into the loop2 surface loop for expression, and the insertion site of NSP4 gene and VP8 antigen gene is T369 of the amino acid sequence shown in SEQ ID NO: 2 -D372, and the NSP4 gene precedes the VP8 antigen gene.
[0030] (3) PP-VP8-NSP4 (parallel expression): insert th...
Embodiment 2
[0035] Expression and purification of recombinant proteins
[0036] The expression vectors of the four antigens constructed in Example 1 were introduced into Escherichia coli (BL21, DE3) to obtain transformed cells. Expression of recombinant protein was induced in E. coli (BL21, DE3) with 0.4 mM isopropyl-β-D-thiogalactoside (IPTG) at 16°C overnight. Take out the induced bacterial solution, centrifuge at 5000rpm at 4°C for 15min, discard the supernatant, resuspend the pellet with 100mL PBS, then perform ultrasonic disruption, ultrasonicate for 10s, stop for 50s, the total ultrasonic time is 1.5h, and then centrifuge again at 13000rpm , 1h, 4 ℃, the supernatant was collected for purification. Pierce for recombinant proteins TMGlutathione agarose (Thermo Fisher Scientific) was used for purification. Take 2 mL of mixed GSTAgarose and add it to the purification column, then add 50 mL of supernatant, mix and combine at room temperature for 2 hours, and discharge the flow-through...
Embodiment 3
[0038] Validation of Purified Antigens
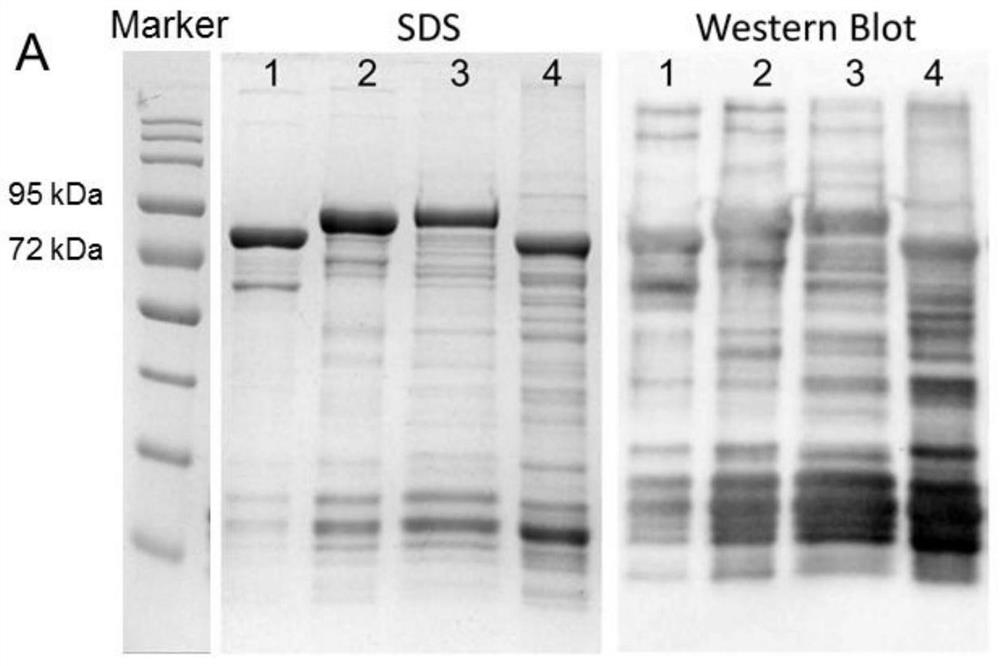
[0039] 1. Verification by polyacrylamide gel electrophoresis: use serially diluted bovine serum albumin (BSA) of different concentrations to conduct SDS-PAGE electrophoresis simultaneously with the sample. The result is as figure 1 shown in the left image.
[0040] 2. Western Blot analysis and verification: After the protein was electrophoresed by 10% SDS-PAGE, it was transferred to 200 mA for 80 min. -P on transfer membrane (Millipore). Detection was performed with rabbit anti-GST antibody (1:5000, Rockland Immunochemicals, Inc., USA) and secondary antibody (1:5000, goat anti-rabbit IgG-HRP, Multi Sciences Biotech, China). After adding an enhanced chemiluminescence substrate (Multi Sciences Biotech, China), the corresponding software Image Lab 5.2.1 was used by Molecular ChemiDoc TM Images were taken by the XRS+ imaging system. The result is as figure 1 shown in the figure on the right.
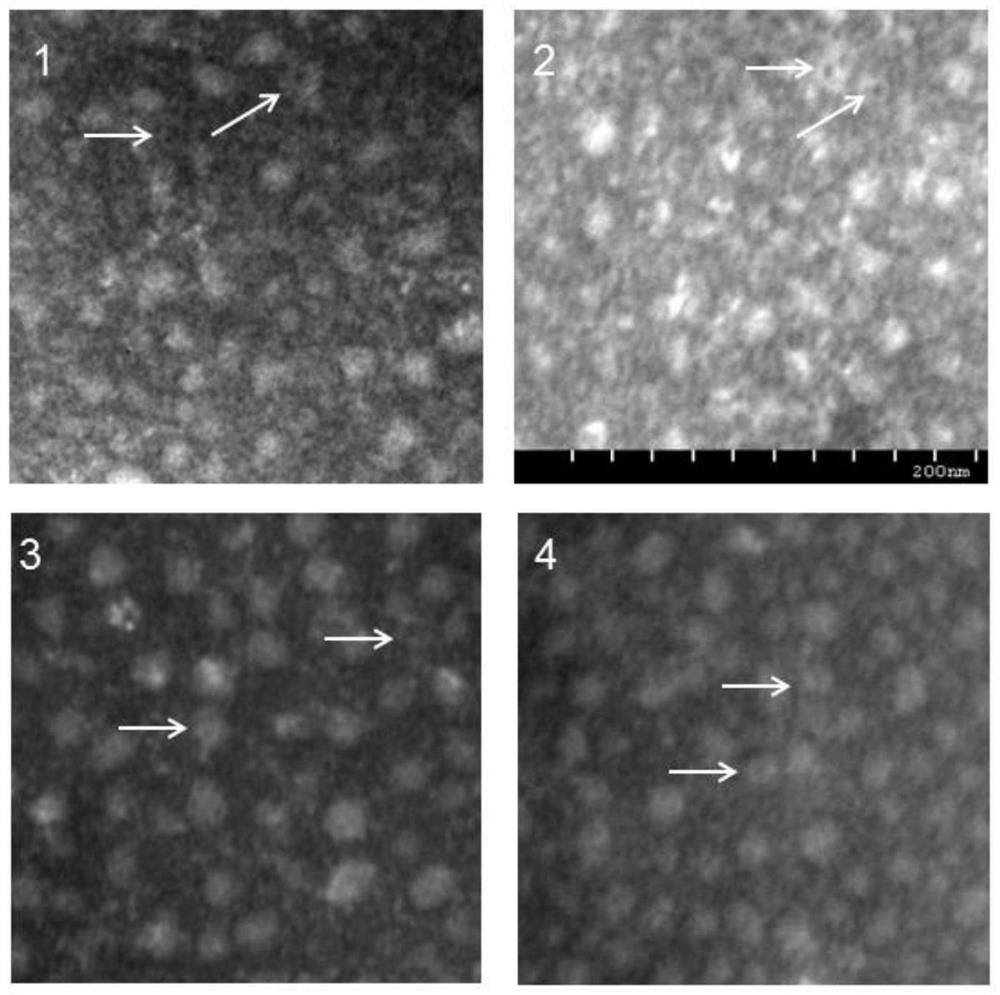
[0041] 3. The morphology of the purif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com