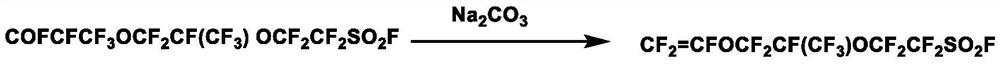Perfluoro (4-methyl-3, 6-dioxa-7-octene) sulfonyl fluoride and preparation method thereof
A technology of dioxa and sulfonyl fluoride, which is applied in the field of perfluorosulfonyl fluoride and its preparation, can solve problems such as unfavorable industrial production, increased amount of "three wastes" and complicated procedures, so as to avoid advanced treatment and reduce Production volume, cost, and effect of simplification of operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
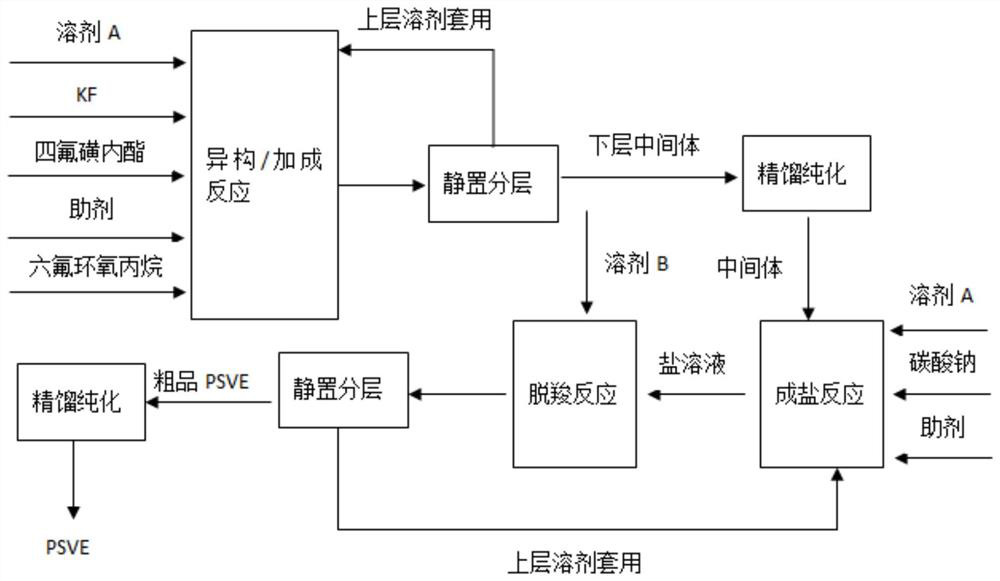
[0029] Intermediate preparation - in a 0.5L autoclave, add 90g of ethylene glycol dimethyl ether and 18g of KF, turn on stirring, cool down to 0°C, then slowly add 180g (1mol) of tetrafluorosultone dropwise, and control the internal temperature to 0°C ~10°C, after the dropwise addition was completed, stirring was continued for 0.5 h, followed by the addition of 0.09 g of dimethylethylenediamine. After the addition was completed, the temperature was lowered to -25°C, and 282.2 g (1.7 mol) of hexafluoropropylene oxide was slowly introduced into the normal pressure. After the reaction was completed, the stirring was stopped and the layers were allowed to stand. Pure intermediate, yield 85%.
[0030] Preparation of intermediate carboxylate solution—in a 1L autoclave, add 256 g of diethylene glycol dimethyl ether, 116.59 g (1.1 mol) of sodium carbonate, stir and heat up to 50 ° C, slowly add 512 g (1 mol) of intermediate dropwise, dropwise After the addition was completed, stirrin...
Embodiment 2
[0033]Intermediate preparation - in a 0.5L autoclave, add 126g of tetrahydrofuran and 18g of KF, turn on stirring, cool down to 0°C, then slowly add 180g (1mol) of tetrafluorosultone dropwise, control the internal temperature from 0°C to 10°C, dropwise After the addition was completed, stirring was continued for 0.5 h, followed by the addition of 0.36 g of tri-n-butylamine. After the addition was completed, it was cooled to -5°C, and 332.0 g (2.0 mol) of hexafluoropropylene oxide was slowly introduced into the normal pressure. After the reaction was completed, the stirring was stopped and the layers were allowed to stand. The upper solvent continued to be applied mechanically, and the lower intermediate was purified by rectification to obtain Pure intermediate, yield 86%.
[0034] Preparation of intermediate carboxylate solution—in a 1L autoclave, add 307g of tetraethylene glycol dimethyl ether, 138g (1.3mol) of sodium carbonate, stir and heat up to 60°C, slowly add 512g (1mol...
Embodiment 3
[0037] Intermediate preparation-in a 0.5L autoclave, add 108g of tetraethylene glycol dimethyl ether and 18g of KF, turn on stirring, cool down to 0°C, then slowly add 180g (1mol) of tetrafluorosultone dropwise, and control the internal temperature to 0 ℃~10℃, continue stirring for 0.5h after the dropwise addition, and then add 0.15g of sodium hydride. After the addition was completed, it was cooled to -15°C, and 298.8 g (1.8 mol) of hexafluoropropylene oxide was slowly introduced into the normal pressure. After the reaction was completed, the stirring was stopped and the layers were allowed to stand. The upper layer solvent continued to be applied mechanically, and the lower layer intermediate was purified by distillation to obtain Pure intermediate, yield 82%.
[0038] Preparation of intermediate carboxylate solution—in a 1L autoclave, add 358 g of tetrahydrofuran and 159 g (1.5 mol) of sodium carbonate, stir and heat up to 55°C, slowly add 512 g (1 mol) of intermediate drop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com