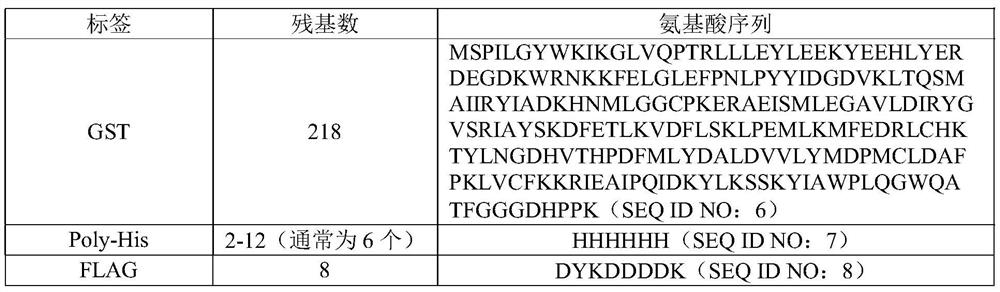
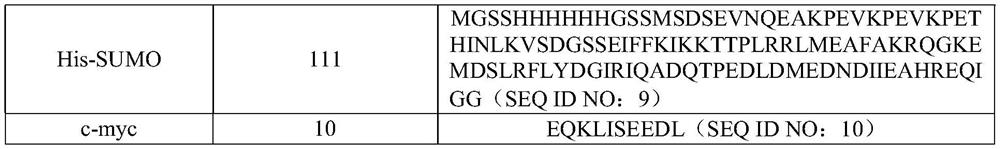
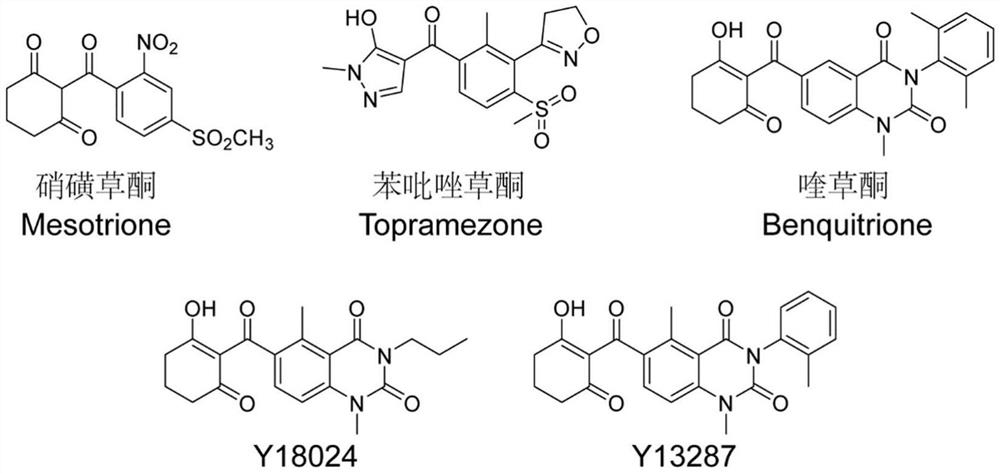
HPPD protein, gene, vector, cell, composition, application of HPPD protein, gene, vector, cell and composition, and method for improving herbicide resistance of crops
A gene and protein technology applied in the field of improving crop herbicide resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Synthesis of coding genes and nucleotide sequences
[0126] The following coding genes and nucleotide sequences were synthesized by Wuhan Jinkarui Bioengineering Co., Ltd. according to the nucleotide sequences provided by the present invention.
[0127] (1) SEQ ID NO: 11
[0128] The gene encoding the protein shown in Table 2 and the nucleotide sequence shown in SEQ ID NO: 11 were synthesized according to the nucleotide sequence shown in SEQ ID NO: 11.
[0129] Table 2
[0130] protein coding gene Mutation site codon SEQ ID NO: 1 WT (wild type) / K418D K418D GAT E423M E423M ATG E423Q E423Q CAG E432M E432M ATG E432I E432I ATT
[0131] (2) SEQ ID NO: 21
[0132] The gene encoding the protein shown in Table 3 and the nucleotide sequence shown in SEQ ID NO: 21 were synthesized according to the nucleotide sequence shown in SEQ ID NO: 21.
[0133] table 3
[0134] protein coding gene Mutatio...
Embodiment 2
[0148] Construction of expression vector
[0149] (1) Amplification of the target gene
[0150] Using the nucleotide sequences of SEQ ID NO: 11, SEQ ID NO: 21, SEQ ID NO: 31, SEQ ID NO: 41, and SEQ ID NO: 51 as templates, respectively, obtained by PCR reaction as shown in Tables 2, 3, and 4 The wild-type and mutant gene sequences of HPPD shown in , 5 and 6 were linked into the corresponding vectors.
[0151] The PCR reaction system and PCR program settings are as follows:
[0152] Table 7
[0153] PCR reaction system composition Addition amount (volume) 10x PCR buffer 5μL 5×dNTPs (10mM) 1μL stencil 1μL forward primer 1.0μL Orientation primer 1.0μL PfuDNA polymerase 0.5μL ddH2O to a total volume of 50 μL
[0154] Table 8
[0155] step temperature(℃) time(s) 1 95 240 2 95 45 3 55 45 4 72 400 5 72 600 6 4 save
[0156] The extension time at 72°C depends on ...
Embodiment 3
[0187] Expression and purification of target proteins
[0188] (1) Expression
[0189] The protein is firstly extracted, and the optimal expression conditions and purification strategies are explored. After the conditions are determined, a large amount of extraction is carried out. Protein expression was carried out in Escherichia coli BL21(DE3) cells (purchased from Kangti Life Technology Co., Ltd., product number KTSM109), and the process is as follows:
[0190] a. Pick a single clone on a plate that has been cultured for 16 hours, add it to 100 mL of LB medium containing the desired antibiotic resistance, and culture at 37 °C and 220 rpm for 5 hours, that is, obvious turbidity is observed;
[0191] b. Expand the small bottle of bacteria in step a into a large bottle of LB medium (containing antibiotics) at a volume ratio of 1:100, and cultivate at 37°C and 220rpm for 3h; wait for OD 600 When the temperature reached 0.7h, the temperature was lowered to 20°C and then 0.2mM ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com