Preparation method of oxybutynin hydrochloride
A technology of oxybutynin hydrochloride and hydroxyphenylacetic acid, which is applied in the field of preparation of oxybutynin hydrochloride, can solve the problems of being unsuitable for industrial production, and the requirement of anhydrous reagents is extremely high, and achieves low cost, low price and stable quality. reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
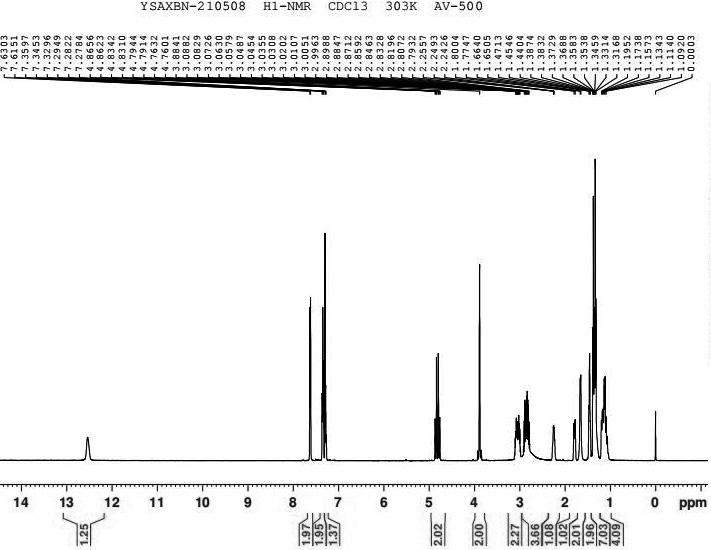
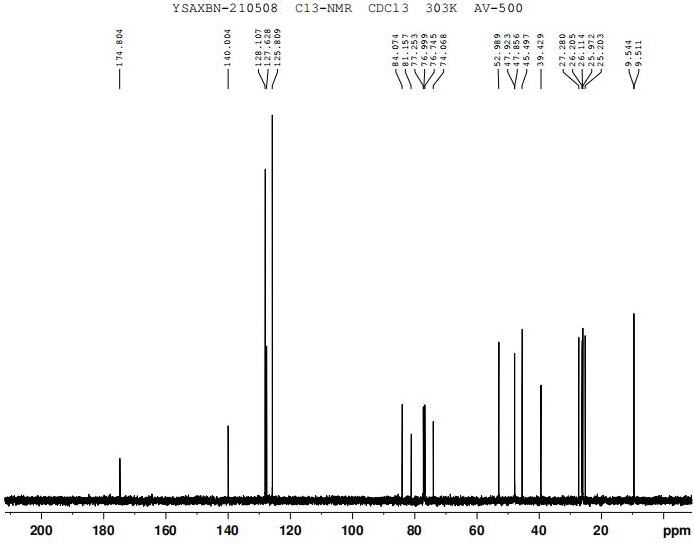
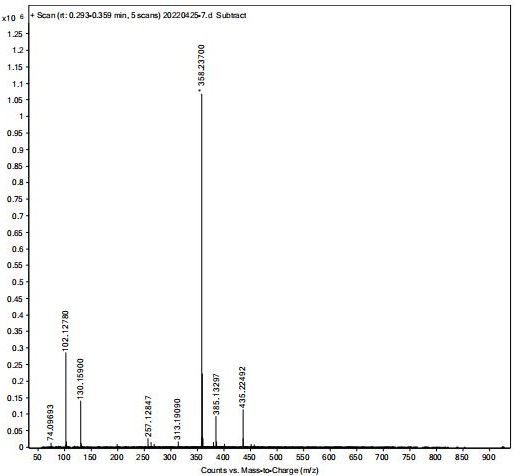
Image
Examples
Embodiment 1
[0032] (1) Synthesis of α-cyclohexyl-α-hydroxyphenylacetic acid propynyl ester (intermediate)
[0033] In a 1000ml three-necked flask, 117.15g (0.5mol) of cyclohexylmandelic acid, 650ml of ethyl acetate, 125g (0.9mol) of potassium carbonate, and 74.51g (1mol) of propyne chloride were sequentially added, and stirred at room temperature (25-35°C) for 25 hours , pour the reactant into 1300 g of cold water, stir for 10 minutes and stand to extract the organic layer, the aqueous layer is extracted once more with 325 ml of ethyl acetate, the organic layers are combined, washed once with water (650 ml), dried over anhydrous magnesium sulfate for 2 hours, filtered, The solvent was evaporated under reduced pressure to obtain a pale yellow oil: 118.3 g, yield: 86.8%.
[0034] (2) Synthesis of free form of oxybutynin
[0035] Add 118g (0.43mol) α-cyclohexyl-α-hydroxyphenylacetate propargyl (intermediate), 34.4g (0.47mol) diethylamine, 43ml 36% formaldehyde solution (0.56mol) to a 500ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com