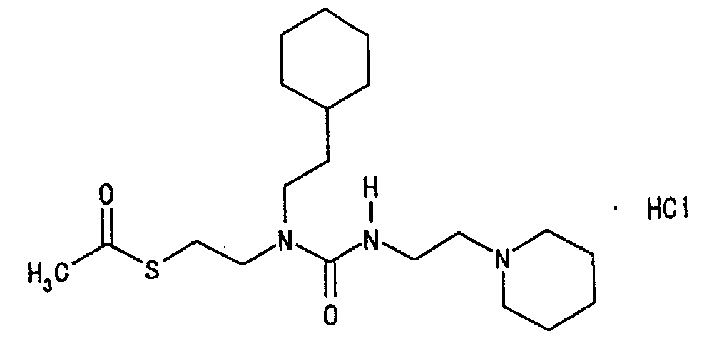
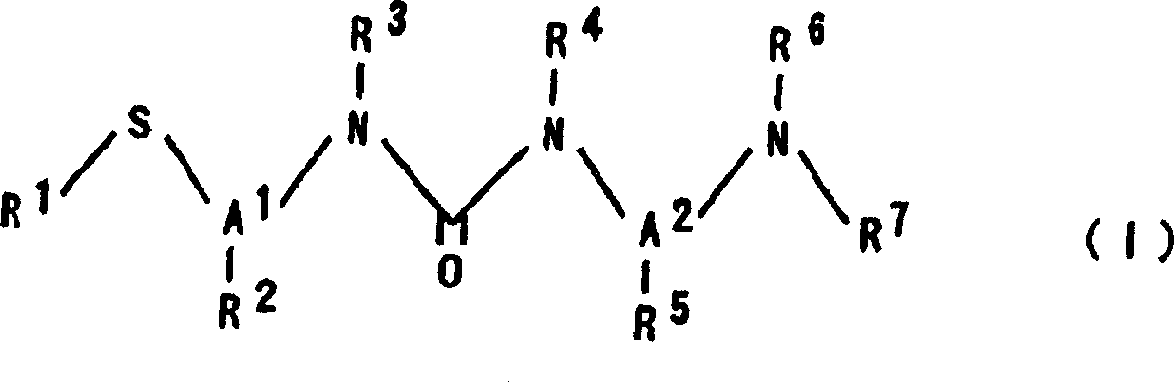
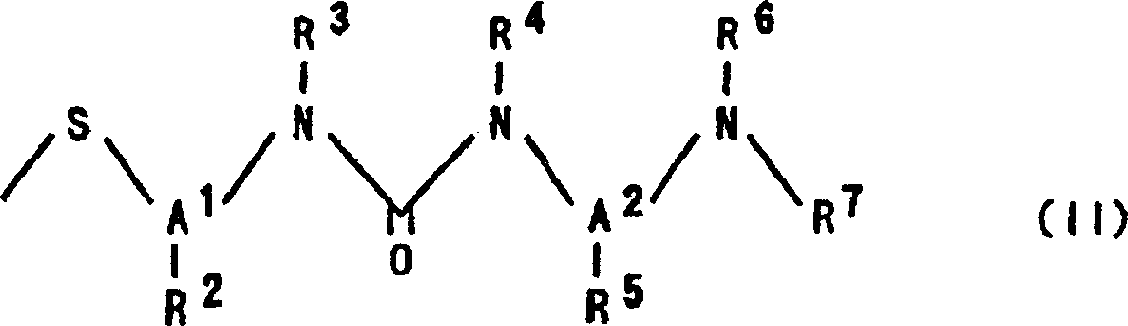
N-substituted-N'-substituted urea derivative and use thereof as TNF-Alpha production inhibitor
A technology of urea derivatives and substituted urea, applied in the field of new N-substituted-N'-substituted urea derivatives, can solve problems such as tissue disorder and deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0088] (1S)-1-Benzyl-2-(4-methyl-piperazin-1-yl)ethanamine (reference compound 1-1)
[0089]
[0090] Under a nitrogen atmosphere, lithium aluminum hydride (531 mg) was suspended in anhydrous ether (14 ml) while ice-cooling, and 1-[(2S)-2-amino-3-phenylpropionyl]-4-methanol was added dropwise A solution of piperazine (1.48g) in anhydrous tetrahydrofuran (7ml). Stir at room temperature for 4 hours. Under ice-cooling, ethyl acetate was slowly added dropwise to the reaction solution until no foaming occurred. Then, 2N aqueous sodium hydroxide solution was added, followed by extraction with chloroform. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain the title compound (reference compound 1-1, 1.25 g).
[0091] [α] D20 +9.1° (c=1.0, chloroform)
[0092] IR (Film, cm-1) 3288, 2936, 2795, 1601, 1495, 1455, 1374, 1283, 1166, 1013
reference example 2
[0094] 3-(1-pyrrolidinyl)propylamine (reference compound 2-1)
[0095]
[0096] N-[3-(1-Pyrrolidinyl)propyl]phthalimide (729 mg) and hydrazine monohydrate (284 mg) were dissolved in methanol (9 ml), and heated under reflux for 2 hours. After cooling, the reaction solution was concentrated under reduced pressure, and 4N aqueous sodium hydroxide solution was added to the residue, followed by extraction with chloroform. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain the title compound (reference compound 2-1, 216 mg).
[0097] IR (Film, cm-1) 3283, 2932, 2874, 2786, 1584, 1460, 1386, 1350, 1293, 1203, 1145, 877
[0098] The following compounds were obtained in the same manner as in Reference Example 2.
[0099] 3-(1-piperidinyl)propylamine (reference compound 2-2)
[0100] IR (Film, cm-1) 3286, 2933, 2853, 1592, 1469, 1442, 1124
[0101] ·[(3RS)-1-Methyl-3-piperidinyl]methylamine (reference compound 2-3)
[0102...
reference example 3
[0107] 2-(1-Homopiperidinyl)ethylamine 2 hydrochloride (reference compound 3-1)
[0108]
[0109] To a solution of N-(tert-butoxycarbonyl)-2-(1-homopiperidinyl)ethylamine (2.31 g) in diethyl ether (5 ml) was added 4N hydrogen chloride in ethyl acetate (24 ml) under a nitrogen atmosphere. After stirring at room temperature for 15 minutes, the precipitated substance was collected by filtration to obtain the title compound as crystals (reference compound 3-1, 1.59 g).
[0110] mp 162~173℃
[0111] IR (KBr, cm-1) 3510, 3384, 2938, 2624, 2045, 1604, 1572, 1463
[0112] The following compounds were obtained in the same manner as in Reference Example 3.
[0113] 2-(4-Methyl-1-piperidinyl)ethylamine 2 hydrochloride (reference compound 3-2)
[0114] mp 155~161℃
[0115] IR (KBr, cm-1) 3477, 3395, 2956, 2630, 1600, 1566, 1502, 1455, 1054, 962
[0116] 2-[4-(Dimethylamino)-1-piperidinyl]ethylamine 3 hydrochloride (reference compound 3-3)
[0117] mp 250℃ and above
[0118] IR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com