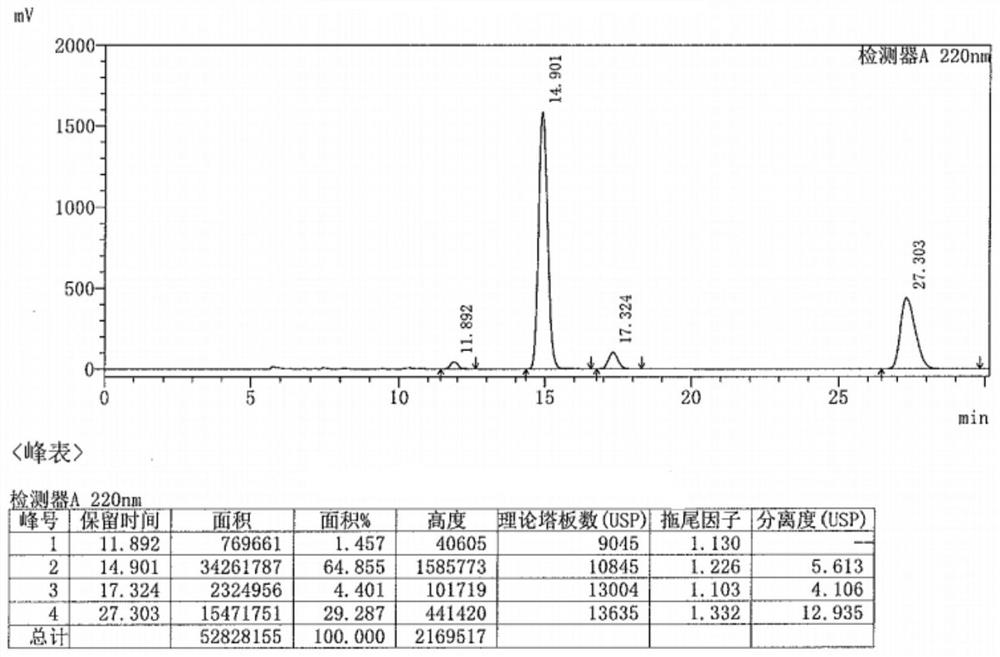
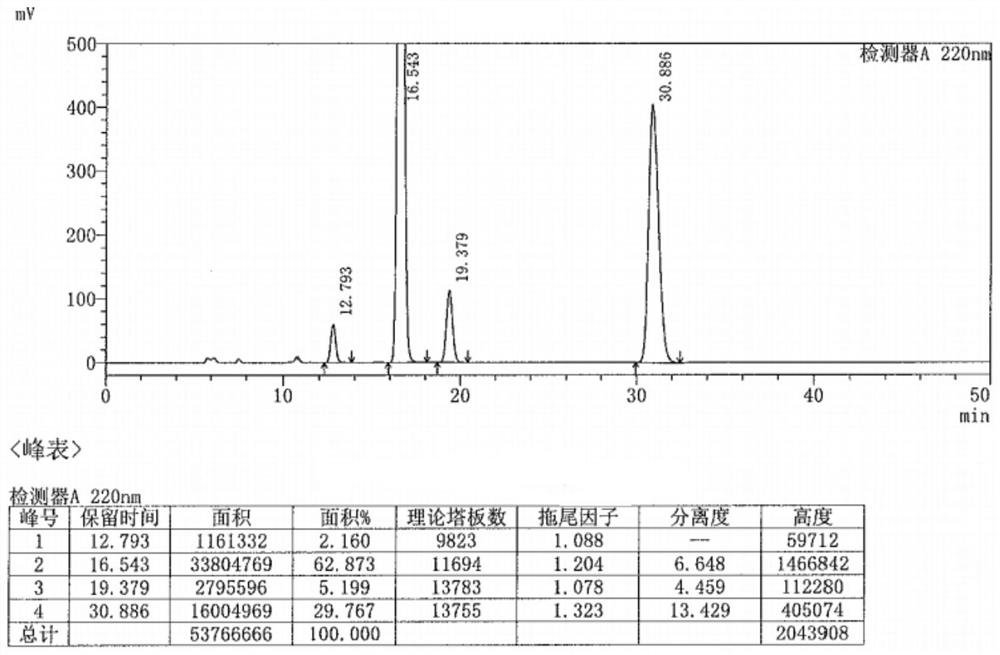
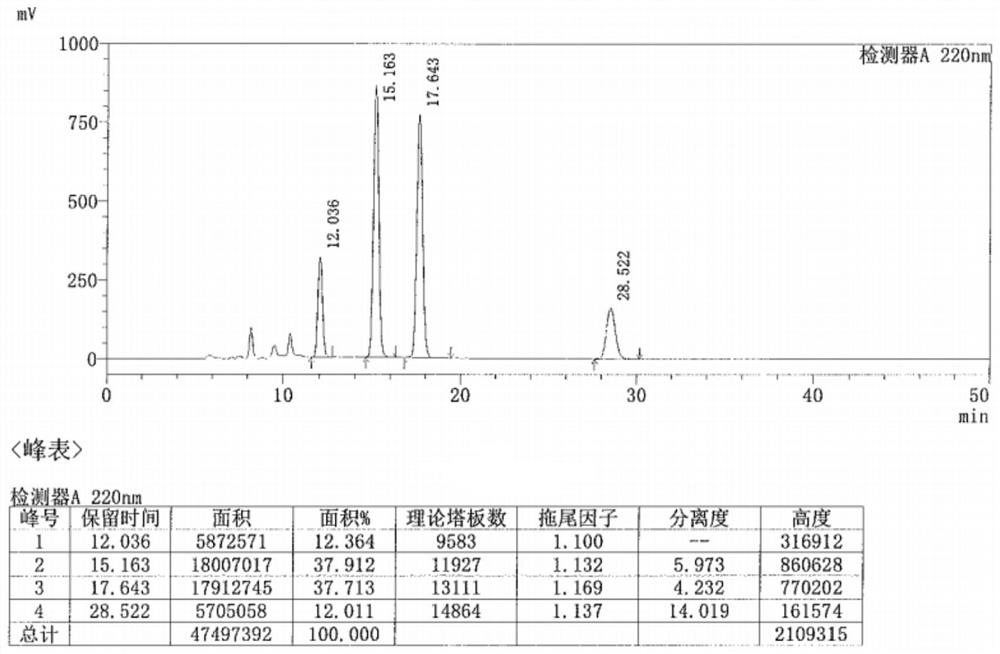
Preparation method of metaraminol bitartrate
A technology for meta-hydroxylamine bitartrate and a compound is applied in the field of preparation of meta-hydroxylamine bitartrate, can solve the problems of high amplification risk, uneasy industrial production, low yield and the like, achieves high enantioselectivity, easy control of reaction operations, The effect of fewer synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of compound VII:
[0037]
[0038] In 1L of ethyl acetate, 100g of L-camphorsulfonic acid, 70g of 2-aminomethylpyridine and an appropriate amount of p-toluenesulfonic acid were respectively added, a water separator was added, and a reflux reaction was performed. g Compound VII, yield 75%.
[0039] Preparation of Compound VI:
[0040]
[0041] In 100 mL of methanol, 10 g of compound VII and 0.2 g of glacial acetic acid were respectively added, the temperature was lowered to -10 to -5 °C under nitrogen atmosphere, 0.4 g of nickel chloride was added, and 2.4 g of sodium borohydride were added in batches. Ethyl ester / n-hexane=5 / 1 was used as the mobile phase for column chromatography to obtain compound VI with a yield of 61%.
Embodiment 2
[0043] Preparation of compound III:
[0044]
[0045] Add 1L absolute ethanol, 16.5g copper acetate monohydrate, 26.5g compound VII to the reaction flask in sequence, stir at room temperature for 10min, then add 100g compound V, lower the temperature to -40~-50°C under nitrogen atmosphere, add 615g nitrile Ethyl ethane, 212g diisopropylethylamine, 1L ethyl acetate was added after the reaction was completed, 1M hydrochloric acid was adjusted to pH 3-4, the layers were separated, the aqueous phase was extracted with ethyl acetate, the organic phases were combined, saturated sodium chloride Washed, dried and concentrated to dryness to give compound III. dr value=2.25:1, ee value=95.6%.
[0046] Preparation of compound II:
[0047]
[0048] Add compound III, 750mL anhydrous methanol, 12g water-containing 10% palladium carbon to the hydrogenation reaction kettle, control the pressure to 4MPa, stir the reaction at 25~30°C to complete the reaction, suction filter, and remove ...
Embodiment 3
[0052] Preparation of compound III:
[0053] 100 mL of absolute ethanol, 1.65 g of copper acetate monohydrate, and 2.65 g of compound VI were sequentially added to the reaction flask, stirred at room temperature for 10 min, then 10 g of compound V was added, the temperature was lowered to -40 to -50 °C under a nitrogen atmosphere, and 61.5 g of Nitroethane, 21.2g diisopropylethylamine, 100mL ethyl acetate was added after the reaction was completed, 1M hydrochloric acid was adjusted to pH 3-4, the layers were separated, the aqueous phase was extracted with ethyl acetate, the organic phases were combined, saturated chlorine washed with sodium chloride, dried and concentrated to dryness to obtain compound III. Forward HPLC detection, dr value = 2.13:1, ee value = 93.4%. Preparation of compound II:
[0054] Add the residue compound III of the previous step, 75 mL of anhydrous methanol, 1.2 g of 10% palladium carbon with water to the hydrogenation reaction kettle, control the pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com