Fudosteine inhalation solution preparation as well as preparation method and application thereof
A technology of fudosteine and solution, which is applied in the field of fudosteine inhalation solution preparation and its preparation, can solve the problem of aerosol drug concentration and particle size distribution research, nebulized inhalation preparation treatment effect needs to be improved, inhalation agent gas Problems such as unclear mist generation characteristics, etc., to achieve the effect of excellent drug effect, excellent drug concentration, and excellent expectorant drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
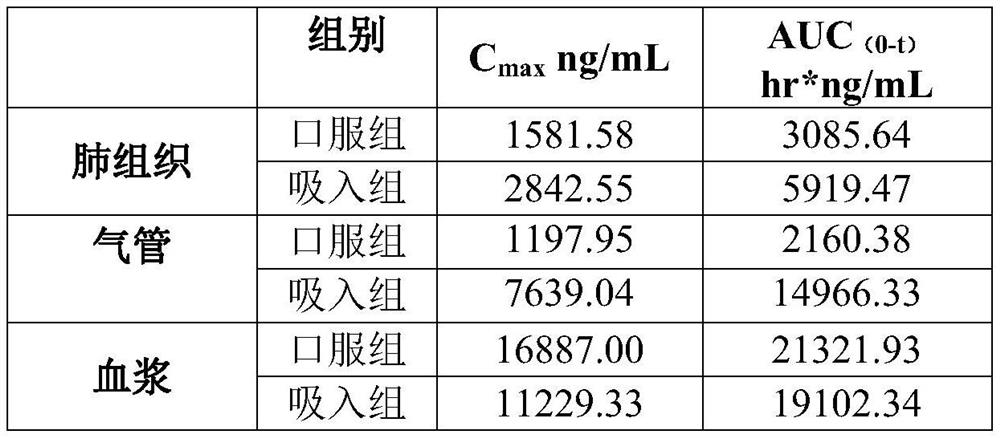
Image
Examples
Embodiment 1
[0068] Embodiment 1, the preparation of solution preparation of fodosteine inhalation of the present invention
[0069] Add fodosteine to a certain amount of water for injection, stir until it is completely dissolved, and then make up to volume with water for injection. The dosage of each ingredient is shown in Table 1 below. The concentration of fadosteine in the obtained solution preparation for fadosteine inhalation was 30 mg / ml.
[0070] Table 1
[0071] Element effect Dosage fodosteine active substance 30g Water for Injection solvent to 1000ml
Embodiment 2
[0072] Embodiment 2, the preparation of solution preparation of fodosteine inhalation of the present invention
[0073] Add fodosteine to a certain amount of water for injection, stir until it is completely dissolved, and then make up the volume with water for injection. The dosage of each ingredient is shown in Table 2 below. The concentration of fadosteine in the obtained solution preparation for inhalation of fadosteine was 250 mg / ml.
[0074] Table 2
[0075] Element effect Dosage fodosteine active substance 250g Water for Injection solvent to 1000ml
Embodiment 3
[0076] Embodiment 3, the preparation of solution preparation for inhalation of fodosteine of the present invention
[0077] Add fodosteine to a certain amount of water for injection, stir until it is completely dissolved, and then use water for injection to make up the volume. The dosage of each ingredient is shown in Table 3 below. The fadosteine concentration in the obtained solution preparation for fadosteine inhalation was 40 mg / ml.
[0078] table 3
[0079] Element effect Dosage fodosteine active substance 40g Water for Injection solvent to 1000ml
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com