Catalyst for hydrolysis hydrogen production photocatalytic reaction and preparation method thereof
A photocatalytic reaction and catalyst technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve the problems of performance failure, agglomeration, disadvantage, etc. Effects on performance and cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] TiO 2 (B) synthesis steps are as follows:
[0054] 9 mL of ethylene glycol was transferred to an 80 mL Teflon-lined stainless steel autoclave.
[0055] Then, 0.3 mL of TiCl was added at room temperature 4 It was gradually added dropwise to the suspension until no HCl gas formed at room temperature. After that, an equal volume of deionized water was added to the mixture. The sealed autoclave was heated in an oven at 150 °C for 4 hours.
[0056] Finally, the resulting TiO 2 (B) The nanoflower product was collected by centrifugation, washed with deionized water and absolute ethanol, and dried in a vacuum oven at 60 °C for 24 h.
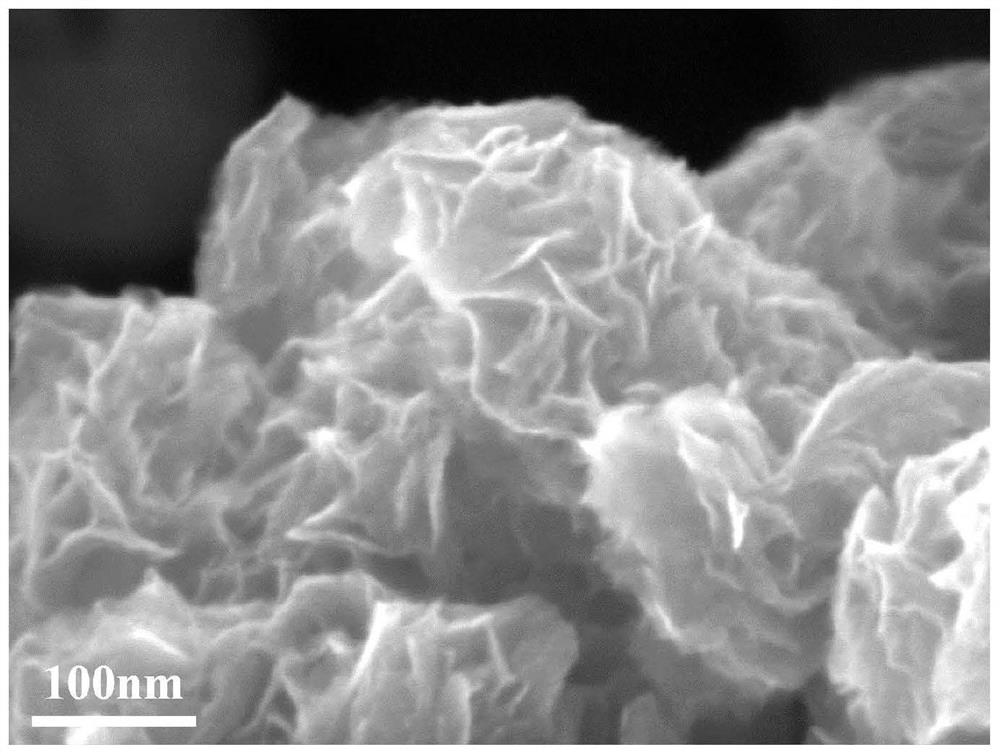
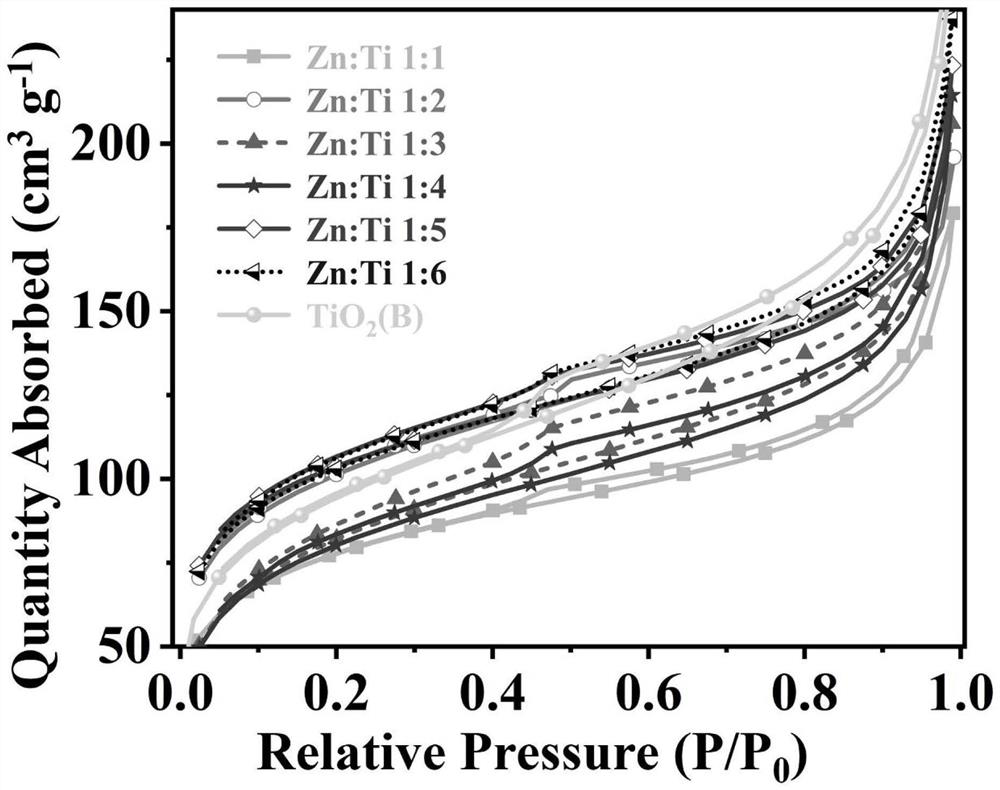
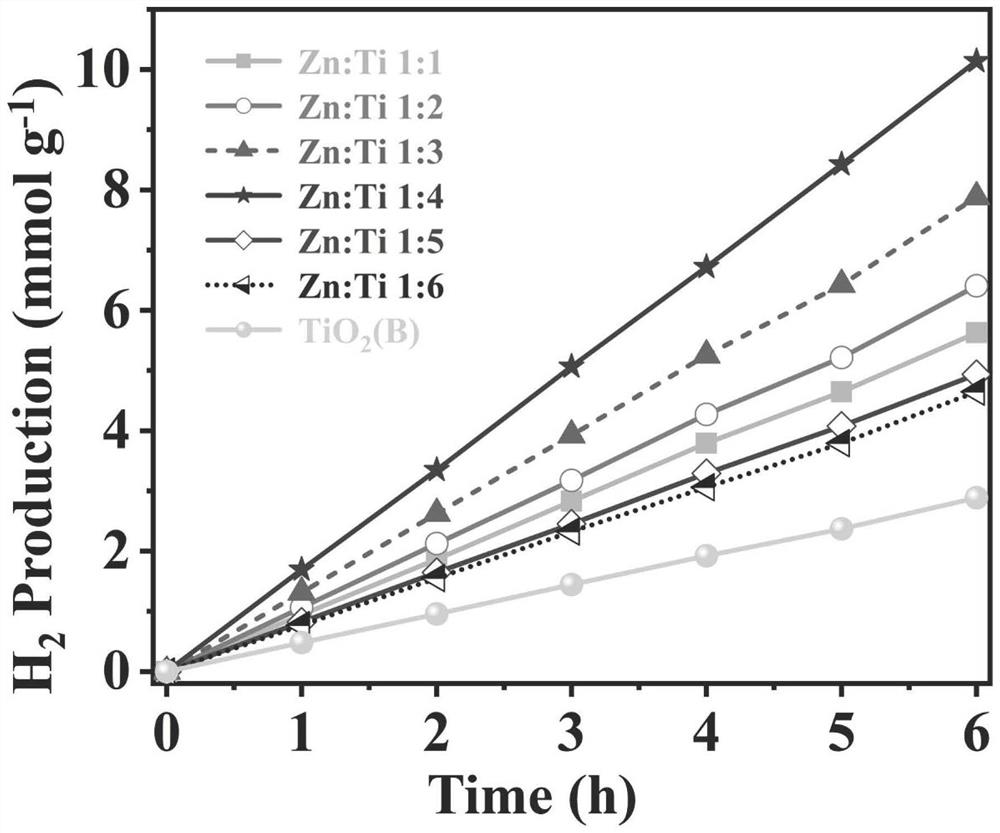
[0057] It was determined that TiO 2 (B) The specific surface area of the nanoflower is 395.574m 2 / g, the photocatalytic hydrogen production rate was 0.482 mmol / g / h.
Embodiment 2
[0059] The molar ratio of Zn:Ti in the catalyst is 1:1, and the synthesis steps are as follows:
[0060] (1) Preparation of ZnO nanorods:
[0061] In a containing 0.05mol / L Zn(NO 3 ) 2 And 0.05mol / L urotropine mixed aqueous solution was carried out in a quartz electrolytic cell, and the quartz electrolytic cell was placed in a water bath environment of 90 ℃ for electrodeposition.
[0062] Then, the white product was collected by centrifugation, washed several times with deionized water and absolute ethanol, and dried at 60 °C overnight to obtain ZnO nanorods.
[0063] Among them, CFs, platinum plate and saturated calomel electrode (SCE) were used as working electrode, counter electrode and reference electrode, respectively; the voltage on the working electrode was negative 1.1v, and the reaction time was 2h.
[0064] Before the preparation of CFs working electrodes, CFs were separately placed in acetone, deionized water and ethanol for ultrasonic treatment.
[0065] (2) Pr...
Embodiment 3
[0071] In the catalyst, the mol ratio of Zn:Ti is 1:2, and the synthesis step is the same as in Example 2, and the difference is:
[0072] Weigh 0.6mL TiCl 4 Gradually drop into 18 ml of the ethylene glycol suspension.
[0073] It has been determined that the specific surface area of nanoflowers with a Zn:Ti molar ratio of 1:2 is 352.465m 2 / g, the photocatalytic hydrogen production rate was 1.064 mmol / g / h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com