In-vitro dissolution method of mirabegron sustained release tablet
An in vitro dissolution and mirabegron technology, applied in the direction of testing pharmaceutical preparations, instruments, measuring devices, etc., can solve the problems of no high discrimination, inability to truly simulate the absorption trough conditions to reflect the release in vivo, and one-sided differences, etc., to achieve Save R&D costs, reduce R&D risks, and improve the success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Optimize the dissolution medium.
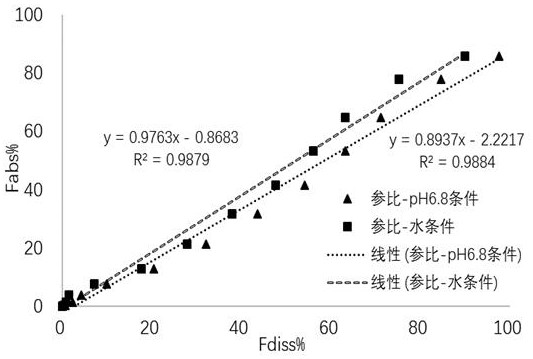
[0072] Mirabegron sustained-release tablet reference preparation was under two different media conditions of water and pH 6.8 phosphate buffer, and the flow rate of the dissolution medium was 4 mL / min; The dissolution medium was internally circulated at a flow rate of 100 mL / min for 8 h, and the dissolution test was conducted over a period of 8 h.
[0073] The concentration of mirabegron samples at each time point was detected by high performance liquid chromatography, and the dissolution curves and in vitro and in vivo correlation curves were obtained.
[0074] The test results are as Figure 1~2The test results show that at 480 min, the cumulative dissolution rates of the reference preparation under two different dissolution media conditions of water and pH 6.8 phosphate buffer are 89.87% and 97.50%, respectively. The dissolution rate was closer to the in vivo absorption rate (85.73%). From the in vitro and in vivo correlation cur...
Embodiment 2
[0076] The dissolution medium flow rate is optimized.
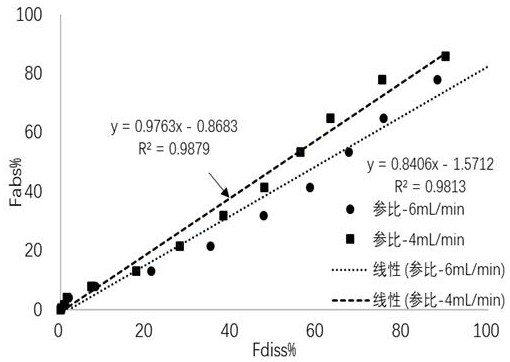
[0077] According to the conclusion of Example 1, when water is used as the dissolution medium, Mirabegron sustained-release tablets show better in vitro and in vivo correlation. Therefore, in this example, water is used as the dissolution medium, and the flow rate of the dissolution medium is 4 Or 6 mL / min, the dissolution process is the natural swelling in the 0~1.5 h, the internal circulation of the dissolution medium at the flow rate of 100 mL / min in the 1.5~8 h, and the dissolution test is carried out for 8 h. Correlation.
[0078] The test results are as Figure 3~4 As shown, the test results show that at 480 min, the cumulative dissolution rates of the reference preparation under the two different conditions of dissolution medium flow rate of 4 mL / min and 6 mL / min are 89.87% and 100.15%, respectively. At 4 mL / min, the cumulative dissolution rate was closer to the in vivo absorption rate (85.73%). From the in vitr...
Embodiment 3
[0080] According to the optimal test conditions of Examples 1 and 2, the dissolution process was further optimized.
[0081] The reference preparation of mirabegron sustained-release tablets used water as the dissolution medium, and the flow rate of the dissolution medium was 4 mL / min. The effects of different dissolution processes on the correlation between in vitro and in vivo were compared;
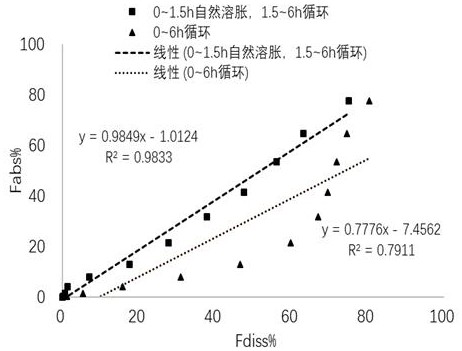
[0082] First, tests were performed under two dissolution conditions:
[0083] A) The dissolution medium is internally circulated at a flow rate of 100 mL / min from 0 to 6 h;
[0084] B) Natural swelling in the 0~1.5 h, and internal circulation of the dissolution medium at a flow rate of 100 mL / min in the 1.5~6 h.
[0085] The test results are as Figure 5 As shown, at 360 min, the cumulative dissolution percentages in vitro under the dissolution conditions of A) and B) were 80.32% and 75.10%, respectively, both of which were close to the in vivo absorption percentage (77.82%) at this ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com