Amino lipid as well as preparation method and application thereof
An amino lipid and hydrocarbon-based technology, applied in the field of medicinal chemistry, can solve the problems of cytotoxicity and low efficiency, and achieve the effects of low equipment requirements, high atom economy, and enhanced escape ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
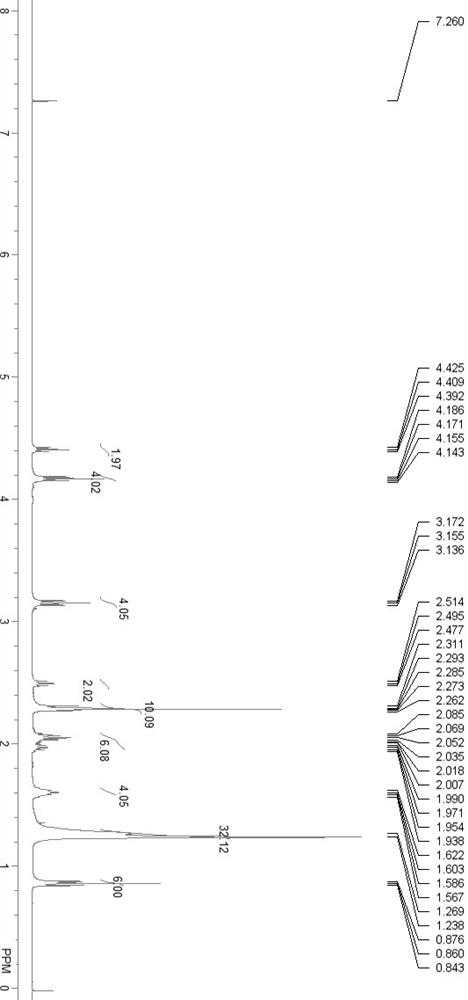
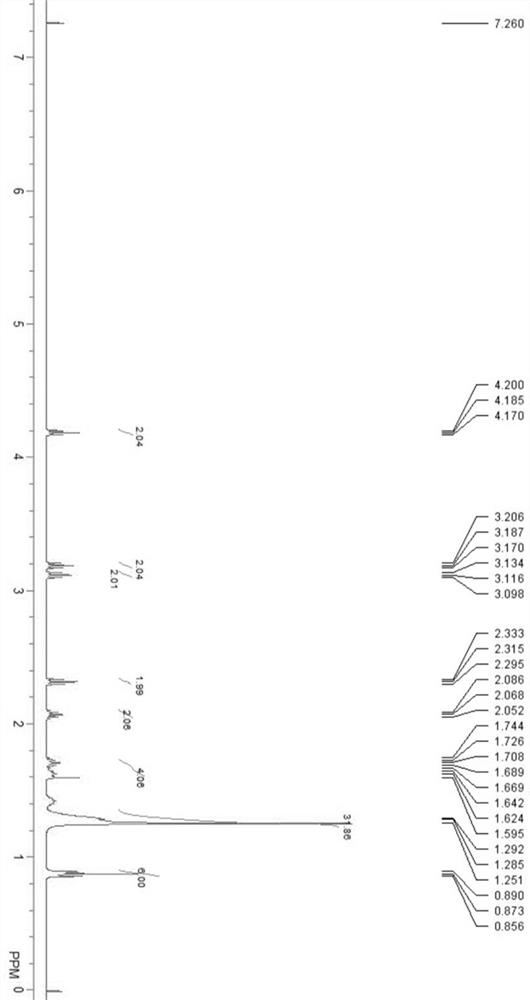
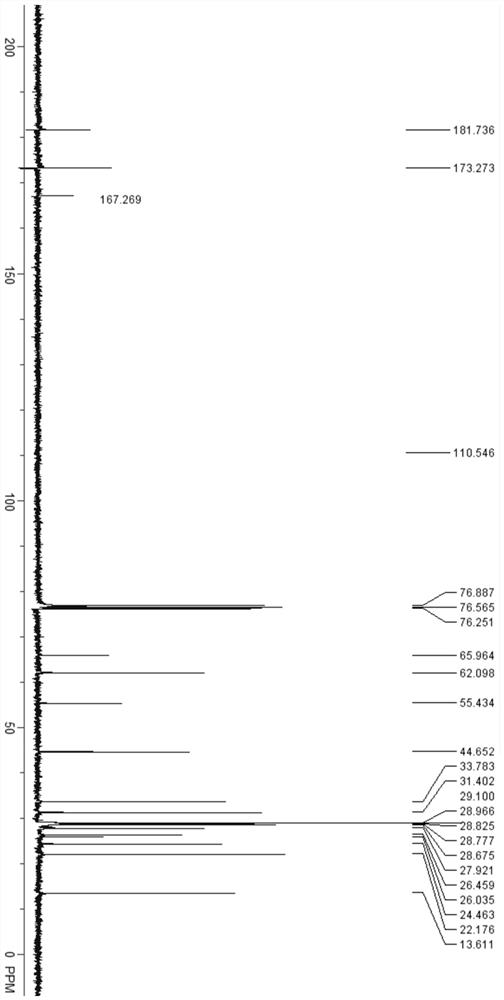
Image
Examples
Embodiment 1
[0070] Example 1 as G as G 1 G 2 Selected from-O-C (= O) -The parallel synthesis and representative
[0071]
[0072] Add 3-cymbal-1-propitol (173 μl, 2mmol), Dipea (442 μl, 2.4 mmol) to the THF, -20 ° C in a 50ml reaction bottle (184 mg, 1 mmol) and 10ml. Stir at room temperature to react overnight. You get STEP I solution (1mmol / 10ml), add laurel acid (481mg, 2.4 mmol), EDC.HCL (460mg, 2.4 mmol), DMAP (6mg, 0.05 mmol), and Dipea (442μL, 2.4mmol, 2.4mmol ), Stir at room temperature for 6 hours, and get 0.1 m STEP II solution (1mmol / 10ml).
[0073] Use a pipette to transfer the Step II solution to the 96 -hole plate of 1.5 ml (0.1 ml, 0.01 mmol), each hole, and the THF solution (0.1 ml, 0.02 mmol, 0.2 0.2 of the alcohol (0.1 ml, 0.02 mmol, 0.2 M) Stir at room temperature for 6h, TLC detects STEP II raw materials. After the reaction is over, the normal temperature is volatilized until the basic solvent, that is, the 16 amino lipid compounds are obtained. For mass spectrometry, se...
Embodiment 2
[0077] Example 2 as G 1 G 2 Different from each other from -CH 2 -He-O-C (= O)-Time, the parallel synthesis and representation of the amino lipid compound library of the OX series
[0078]
[0079] Add Sixteen alkyl sulfurnol (308 μl, 1mmol), Dipea (221 μl, 1.2mmol), and 10ml of THF, -20 Add 3-cymbal-1-propylene (87 μl, 1mmol) and Dipea (221 μl, 1.2mmol) after 30 minutes of stirring reaction for 30 minutes. . HCL (230mg, 1.2mmol), DMAP (3mg, 0.025mmol), and Dipea (221 μl, 1.2mmol), stir 6h at room temperature, and get 0.1 m Step II solution (1mmol / 10ml).
[0080] Use a pipette to transfer the Step II solution to the 96 -hole plate of 1.5 ml (0.1 ml, 0.01 mmol), each hole, and the THF solution (0.1 ml, 0.02 mmol, 0.2 0.2 of the alcohol (0.1 ml, 0.02 mmol, 0.2 M) Stir at room temperature for 6h, TLC detects STEP II raw materials. After the reaction is over, the normal temperature is volatilized until the basic solvent, that is, the 16 amino lipid compounds are obtained. For mass s...
Embodiment 3
[0084] Example 3: When G 1 G 2The same on-O-C (= O)-When the DX series, one of the DX series of the parallel synthesis and representation of the amino lipid compound library
[0085]
[0086] Add 6-pyramid-1-hexol (274 μl, 2mmol), Dipea (442 μl, 2.4mmol) when adding a three-gipedis (184 mg, 1 mmol) and 10ml of THF, -20 ° C. Stir at room temperature to react overnight. You get the STEP I solution (1mmol / 10ml), and add cricket acid (463 μl, 2.4 mmol), EDC.HCL (460mg, 2.4 mmol), DMAP (6mg, 0.05mmol), and Dipea (442 μL, 2.4mmol, 2.4mmol ), Stir at room temperature for 6 hours, and get 0.1 m STEP II solution (1mmol / 10ml).
[0087] Use a pipette to transfer the Step II solution to the 96 -hole board of 1.5 ml (0.1 ml, 0.01 mmol), and each hole with THF solution (0.1 ml, 0.02 mmol, 0.2m) with THF in each hole (0.1 ml, 0.02 mmol, 0.2m) Stir at room temperature for 6h, TLC detection does not have Stepii raw materials. After the reaction is over, the normal temperature is volatilized unti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com