Preparation method of S-alkyl-L-cysteine sulfoxide with high purity and high conversion rate
A technology of cysteine sulfoxide and high conversion rate, which is applied in the field of preparation of high-purity and high-conversion S-alkyl-L-cysteine sulfoxide, which can solve problems such as unstable applications and achieve simplified methods , save time, and achieve a single effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
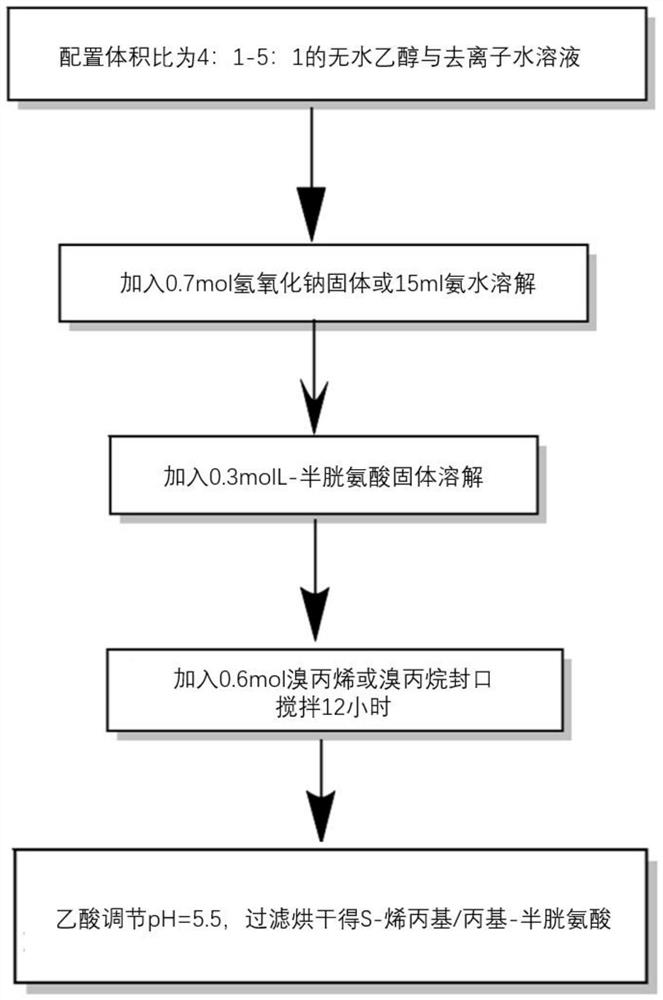
[0047] like figure 1 As shown, it is a flow chart of the first step of synthesizing S-alkyl-L-cysteine sulfoxide in the present invention.
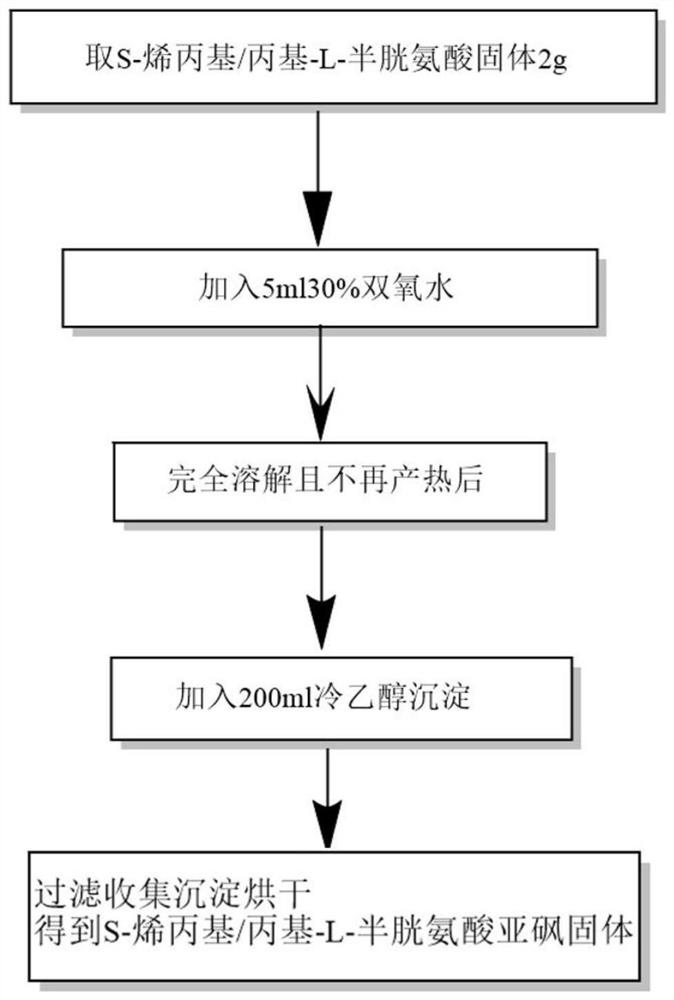
[0048] like figure 2 As shown, it is the flow chart of the second step in the synthesis of S-alkyl-L-cysteine sulfoxide.
[0049] The steps for preparing S-allyl-L-cysteine sulfoxide are:
[0050] Step 1: Mix 200ml of absolute ethanol and 40ml of deionized water in a beaker, then weigh 0.7mol of sodium hydroxide particles or 15ml of ammonia water, and then weigh 0.3mol of L-cysteine after the rotor is fully stirred and dissolved. The solid was added to the system, and after it was completely dissolved, 0.6 mol of bromopropene was added and stirred for a while to obtain a transparent and clear pale yellow solution, which was sealed with a sealing film and continued to stir overnight for about 12 hours. Then adjust the pH of the solution to 5.5 with acetic acid. At this time, a large number of white crystals will be precipitated...
Embodiment 2
[0056] Self-made S-alkyl-L-cysteine sulfoxide reacts with cysteine sulfoxide lyase
[0057] Weigh 0.7 g of S-allyl-L-cysteine sulfoxide and S-propyl-L-cysteine sulfoxide respectively, and prepare 3 ml of deionized aqueous solution for each. Add 10 μl of cysteine sulfoxide lyase and 20 μl of substrate to the 1 ml reaction system, react in a water bath at 37°C for 1 min, and use the DNPH method to detect the production of pyruvate to calculate the enzyme activity, such as Figure 4 Shown is the detection result of the reaction between the self-made S-alkyl-L-cysteine sulfoxide and cysteine sulfoxide lyase. It can be seen that the self-made S-alkyl-L-cysteine sulfoxide can be cleaved by cysteine sulfoxide lyase, indicating that the synthesized substance is correct.
Embodiment 3
[0059] HPLC-MS determination of self-made S-alkyl-L-cysteine sulfoxide
[0060] Taking S-allyl-L-cysteine sulfoxide as an example, it was configured into a 30% methanol aqueous solution of 0.5 mg / ml, and then sent to HPLC-MS for identification. The injection volume was 5 μl and the detection wavelength was 214 nm. , the chromatographic column is C18 column, the flow rate is 0.8ml / min, and the ion source is ESI positive ion. Mass spectrometry results such as Figure 5 shown is 177.22 for S-allyl-L-cysteine sulfoxide and 178.22 for positive ion bombardment. It can be seen that the molecular weight is correct, the mass spectrometry result is a single peak, and the molecular weight of each position of this peak is 178.05 as the arrow moves, so the material purity is high.
[0061] The self-made S-propyl-L-cysteine sulfoxide was also subjected to the above operations, and the HPLC-MS identification results were as follows Image 6 As shown, the molecular weight of S-prop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com