Method for rapidly screening vaccine finished products by using dynamic light scattering technology
A dynamic light scattering and vaccine technology, which is applied to the measurement of scattering characteristics, resistance to media-borne diseases, instruments, etc., can solve the problem that the effectiveness and stability of vaccines cannot be quickly screened, the test results of finished vaccines cannot be quantitatively compared, and cannot reflect Inter-batch differences and other issues, to achieve the effect of improving stability and product quality, good stability of test results, and short test cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1-7
[0040] Preparation example 1-7 provides a kind of Japanese encephalitis inactivated virus vaccine, adopts the following method:
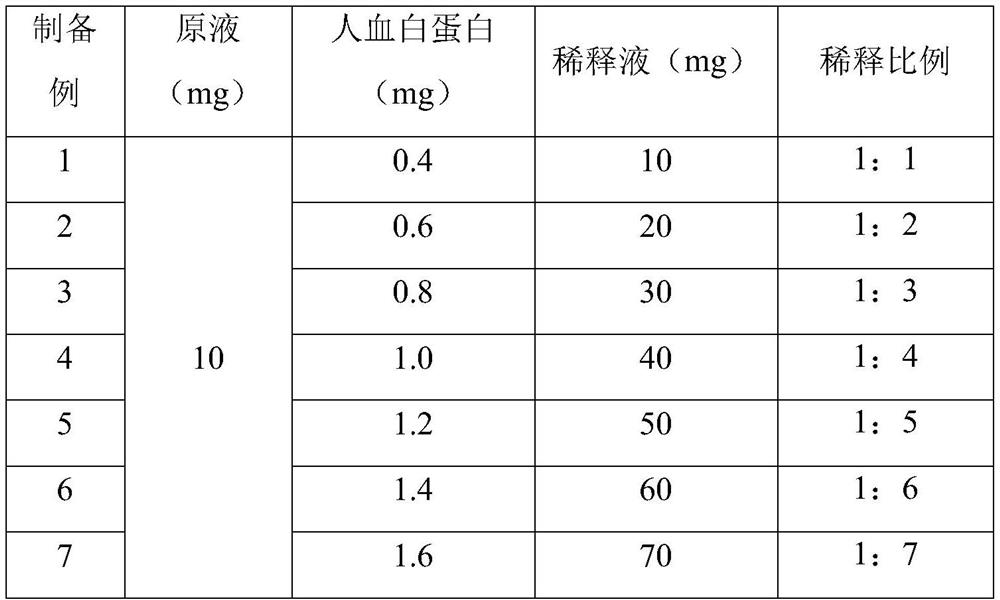
[0041] The difference between the above preparation examples is: in the preparation process of the JE inactivated virus vaccine, the dilution ratio of the JE inactivated virus vaccine stock solution is specifically shown in Table 1.
[0042] The preparation method of JE inactivated virus vaccine is as follows: firstly, weigh human albumin in proportion, dissolve it in PBS solution to obtain a diluent, and control the salt concentration in the diluent to be 0.145mM and pH to be 7-8; then Take 10 mg of the JE inactivated virus vaccine stock solution and mix it with the above dilution to obtain the JE inactivated virus vaccine. Among them, the concentration of human serum albumin in the JE inactivated virus vaccine is 2%.
[0043] Table 1 Dilution ratio of JE inactivated virus vaccine stock solution in Preparation Examples 1-7
[0044]
preparation example 8-10
[0046] Preparation example 8-10 provides a kind of Japanese encephalitis inactivated virus vaccine, adopts the following method:
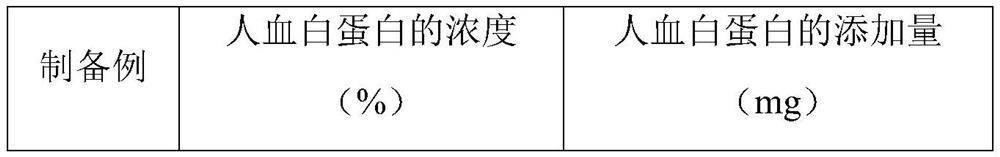
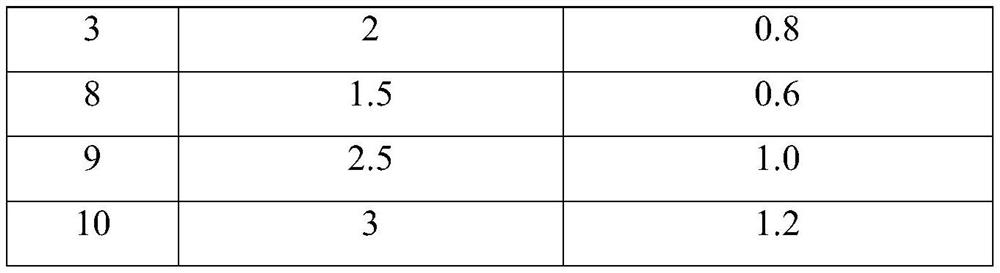
[0047] The difference between the above preparation example and preparation example 3 is: in the preparation process of the JE inactivated virus vaccine, the concentration of human albumin is specifically shown in Table 2.
[0048]The concentration of human albumin in the JE inactivated virus vaccine provided in Table 2 Preparation Example 3 and Preparation Example 8-10
[0049]
[0050]
[0051] Test results
[0052] Detect the JE inactivated virus vaccine provided in Preparation Example 1-10, and measure its particle size and aggregation temperature T respectively. agg Value, potency and thermal stability, and compared the test results.
[0053] Particle size and aggregation temperature T agg Value (the onset temperature of protein aggregation) was measured using a WYATT DynaProPlate Reader III high-throughput protein solution stability ...
Embodiment 1
[0081] Example 1 provides a method for rapidly screening finished vaccine products using dynamic light scattering technology.
[0082] The above method includes the following steps:
[0083] (1) According to the test results of Preparation Examples 1-10, the accelerated thermal stability test and the long-term stability test, the particle size test results of the JE inactivated virus vaccine were correlated with the titer and thermal stability test results to establish a dynamic Light Scattering Database.
[0084] (2) Selecting the finished JE inactivated virus vaccine synthesized at different dilution ratios in the same batch, and using the WYATTDynaPro Plate Reader III high-throughput protein solution stability analyzer to detect the particle size of each JE inactivated virus vaccine product; using The dynamic light scattering database can quickly determine the titer and thermal stability of the JE inactivated virus vaccine under each particle size, and simultaneously detec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com