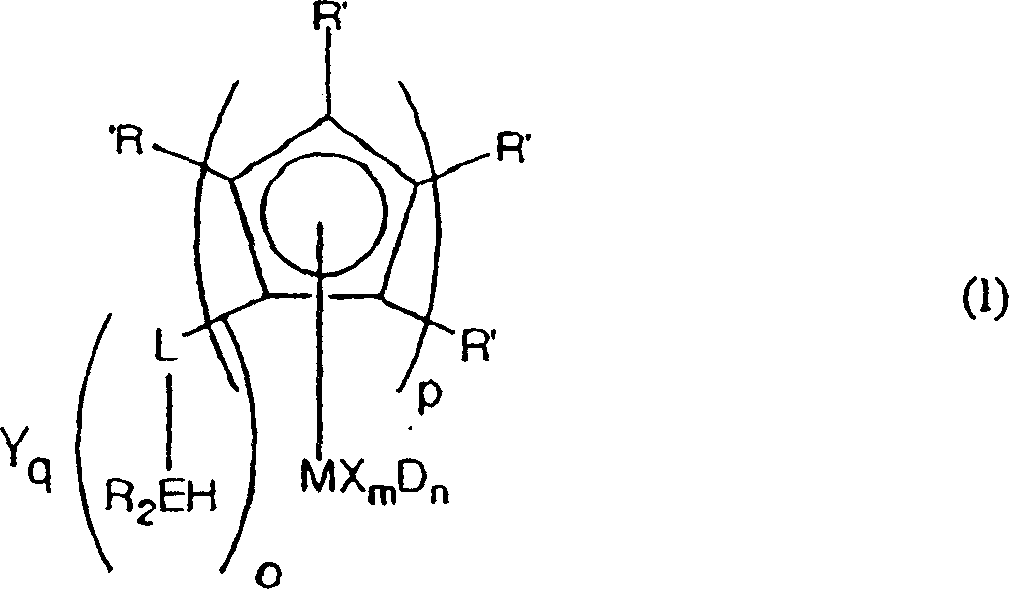
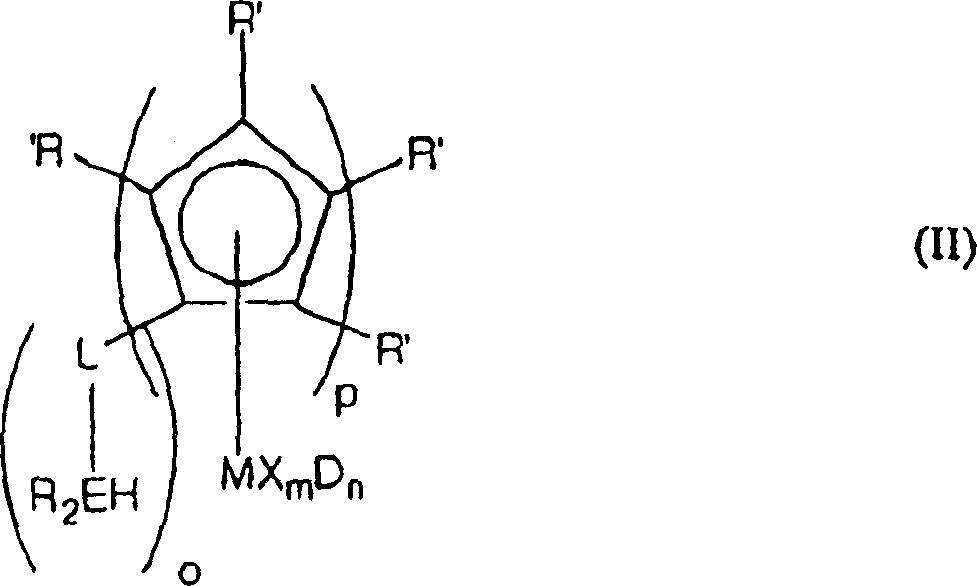
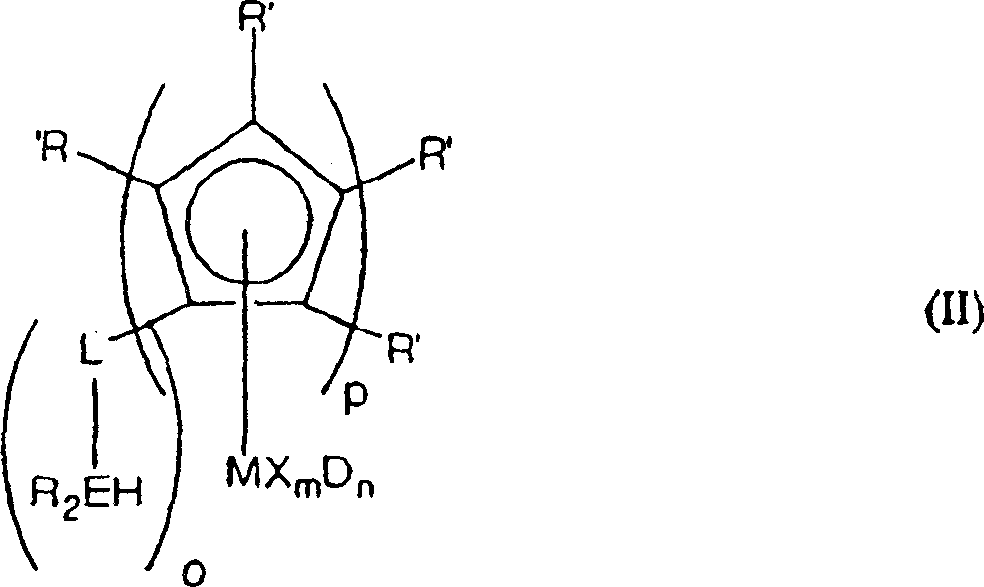
Cyclopentadienyl transition metal compound as polymerization catalysts
A technology based on cyclopentadienyl and transition metals, applied in the direction of metallocene, organic chemistry, chemical instruments and methods, etc., can solve the problem of increasing the final cost of the catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] In this example, a stable isolatable complex was prepared by mixing 2.5 g (8.6 mmol) (2-dimethylaminoethyl) cyclopentadienyl titanium trichloride with 1.2 g (8.6 mmol) 2,6- Prepared by the reaction of lutidine-hydrogen chloride. The reaction was accomplished by first dissolving (2-dimethylaminoethyl)cyclopentadienyltitanium trichloride in 25 mL of dichloromethane, and then adding 2,6-lutidine-hydrogen chloride dry powder. The reaction was carried out at room temperature and continued for 15 minutes. Thereafter, an insoluble red-orange precipitate came out of solution. The red-orange precipitate was isolated by filtration, washed twice with 20 mL of dichloromethane and dried under vacuum. The yield of (2-dimethylaminoethyl)cyclopentadienyl titanium trichloride hydrochloride was 2.7 g (96%).
Embodiment 2
[0107] [ Four (3,5-bis-trifluoromethylphenyl)] sodium borate reaction and preparation. The reaction was carried out by first suspending (2-dimethylaminoethyl)cyclopentadienyl titanium trichloride hydrochloride in 20 mL of dichloromethane, and then adding [tetrakis(3,5-bistrifluoromethylbenzene Base)] sodium borate dry powder and completed. The reaction was carried out at room temperature for 1 hour. Thereafter, a fluorescent yellow solution containing NaCl precipitate was obtained. NaCl was removed by filtration and the solvent was removed from the mother liquor to give a flaky yellow solid. The solid was washed three times with 15 mL of pentane and dried under vacuum. 1.95 g (91%) was recovered to have the formula B[(3,5-CF 3 )C 6 h 3 ] 4 - [2-Me 2 N + (H)CH 2 CH 2 -C 5 h 4 ]TiCl 3 of yellow solid.
Embodiment 3
[0109] In this example, a stable isolatable complex was obtained by mixing 0.20 g (0.6 mmol) (2-dimethylaminoethyl) cyclopentadienyl titanium trichloride hydrochloride with 0.21 g (0.60 mmol) Prepared by the reaction of sodium phenyl borate. The reaction was carried out by first suspending (2-dimethylaminoethyl)cyclopentadienyltitanium trichloride hydrochloride in 15 mL of dichloromethane and then adding sodium tetraphenylborate dry powder. The reaction was carried out at room temperature for 1 hour. Thereafter, a yellow solution containing NaCl precipitate was obtained. NaCl was removed by filtration and the solvent was removed from the mother liquor to give a yellow solid. The solid was washed three times with 15 mL of pentane and dried under vacuum. 0.37 g (99%) was recovered to have the formula B (C 6 h 5 ) 4 - [2-Me 2 N + (H)CH 2 CH 2 -C 5 h 4 ]TiCl 3 of yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com