Thymic peptide fusion protein as one new interferon and its prepn. and use
A technology of fusion protein and thymosin, which is applied in the field of DNA recombination technology and medicine, can solve the problems of cumbersome preparation, separation and purification, and high cost of medicines, and achieve the effect of avoiding cumbersome operations, avoiding duplication of labor, and avoiding high-cost operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Synthesis of IFN-THY fusion gene and construction of expression plasmid in Escherichia coli
[0055] In order to make the IFN-THY fusion protein have the correct spatial conformation, GGGGS was used as the connecting peptide to connect IFN and THY. According to the IFN gene sequence and the nucleotide sequence of the connecting peptide, a pair of primers IFNPRI1 and IFNPRI2 were designed and synthesized, and their nucleotide sequences were:
[0056] IFNPRI1: 5'GCCATATGTG CGATCTGCCT CAAACC 3' (SEQ ID NO: 3)
[0057] IFNPRI2: 5' ATGGATCCAC CACCGCCTTC CTTACTTCTT AAACTTTC 3' (SEQ ID NO: 4)
[0058] Among them, IFNPRI1 is the coding sequence of N-terminal of IFN mature protein; IFNPRI2 is the complementary sequence of IFN C-terminal and connecting peptide coding sequence.
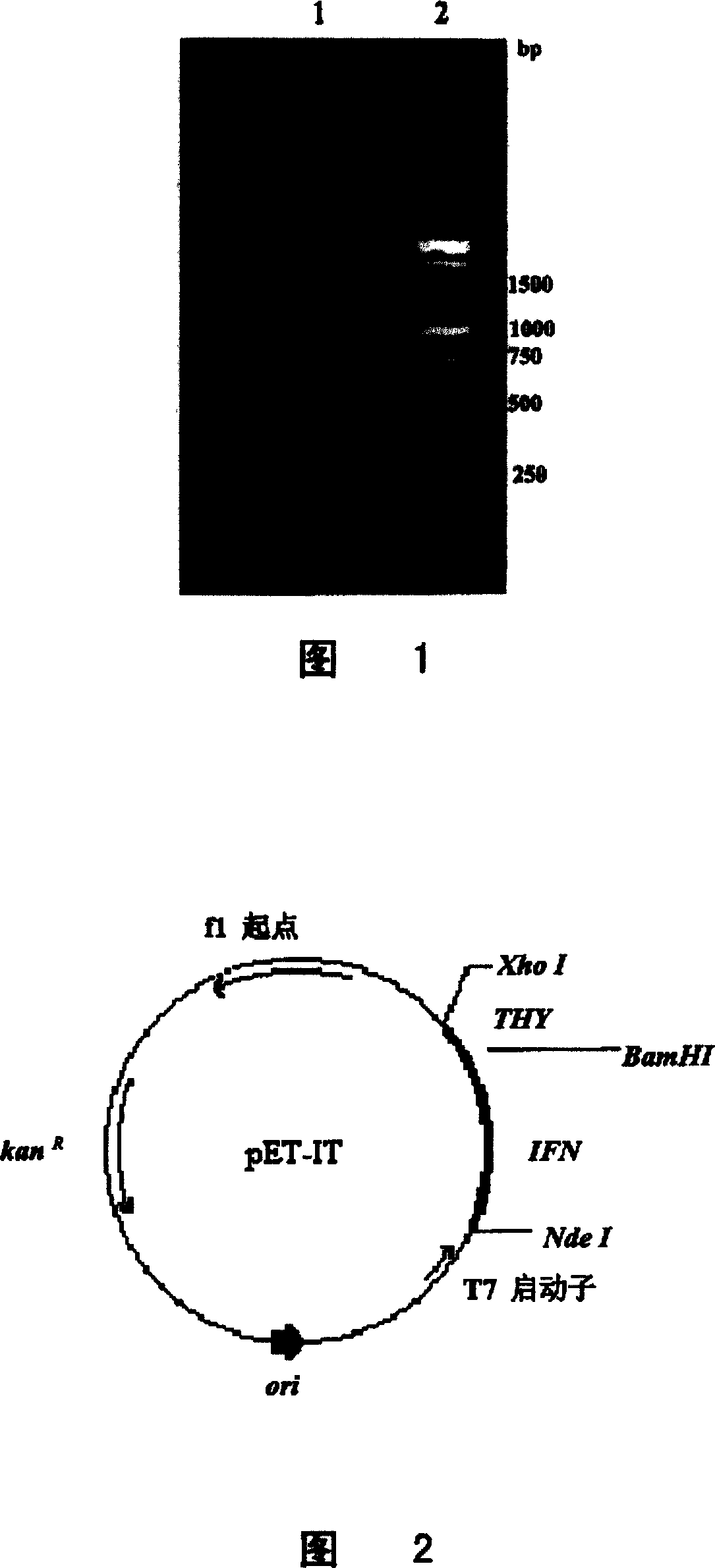
[0059] Using IFNPRI1 and IFNPRI2 as primers and the pSK-IFN plasmid containing IFN gene as a template, PCR amplifies the IFN gene sequence with the connecting peptide nucleotide sequence, and introduces...
Embodiment 2
[0070] Construction of Insect Baculovirus Transfer Plasmid pBacPAK-IT Containing IFN-THY Fusion Gene
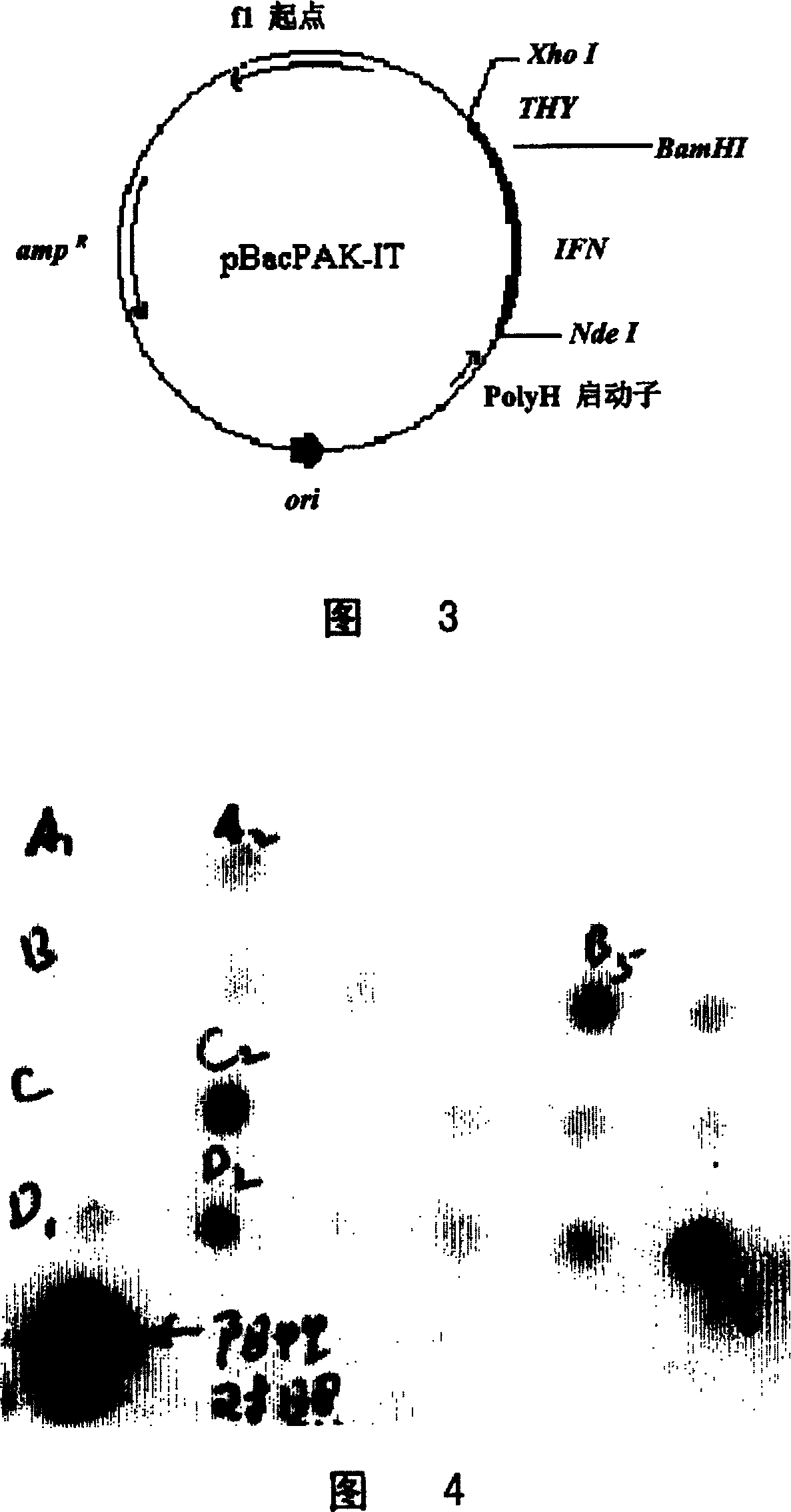
[0071] The Escherichia coli expression plasmid pET-IT containing the IFN-THY fusion gene was first digested with NdeI, then filled in with Klenow enzyme, and then digested with XhoI, and the fusion gene fragment of IFN and THY was separated with low melting point agarose, and mixed with The insect baculovirus transfer plasmid pBacPAK-IT containing the IFN-THY fusion gene was constructed by digesting with BamHI and filling in with Klenow enzyme, and then connecting with pBacPAK8 digested with XhoI (Fig. 3). The plasmid sequentially contains the IFN gene, the GGGGS linker peptide coding sequence, and the THY gene in the direction from 5' to 3'.
Embodiment 3
[0073] Obtaining Recombinant Insect Baculovirus Containing IFN-THY Fusion Gene
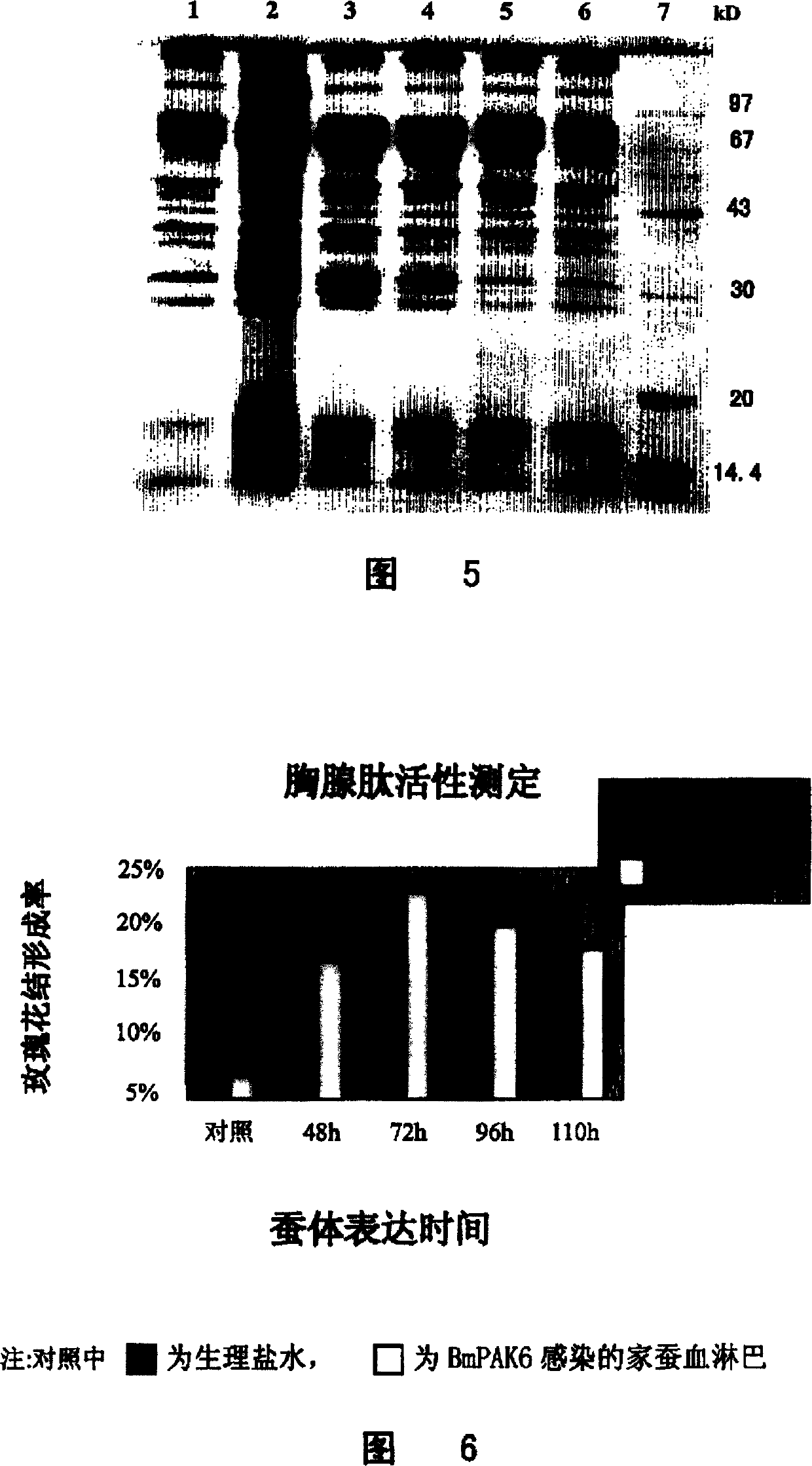
[0074] Take 5 microliters of insect baculovirus transfer plasmid pBacPAK-IT containing IFN-THY fusion gene and 6 microliters of modified virus BmBacPAK6 linearized by Bsu36I digestion, add 100 microliters of serum-free TC-100 medium and mix well. Add 6 microliters of Lipofectin to 100 microliters of serum-free TC-100 medium and mix well. Mix the two evenly, place at room temperature for 15 minutes, add 800 microliters of serum-free TC-100 medium and mix well. Wash Bm N cells previously cultured in a 35mm culture dish twice with serum-free TC-100 medium, and add the transfer plasmid and Lipofectin mixture dropwise, culture at 27°C for 4-5 days, collect the supernatant for the first round Plaque screening. Take 5 microliters of supernatant to infect Bm N cells in a 35mm culture dish, discard the supernatant after 1 hour and add equal volumes of mixed TC-100 medium and low melting point agarose. P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com