Process for synthesizing lithium cobalt oxide as positive electrode material
A technology of lithium cobalt oxide and synthesis method, which is applied in the direction of battery electrodes, alkaline storage battery electrodes, electrical components, etc., can solve the problems of prolonging calcination time, large loss of first discharge capacity, and difficulty in industrial production, so as to improve cycle stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
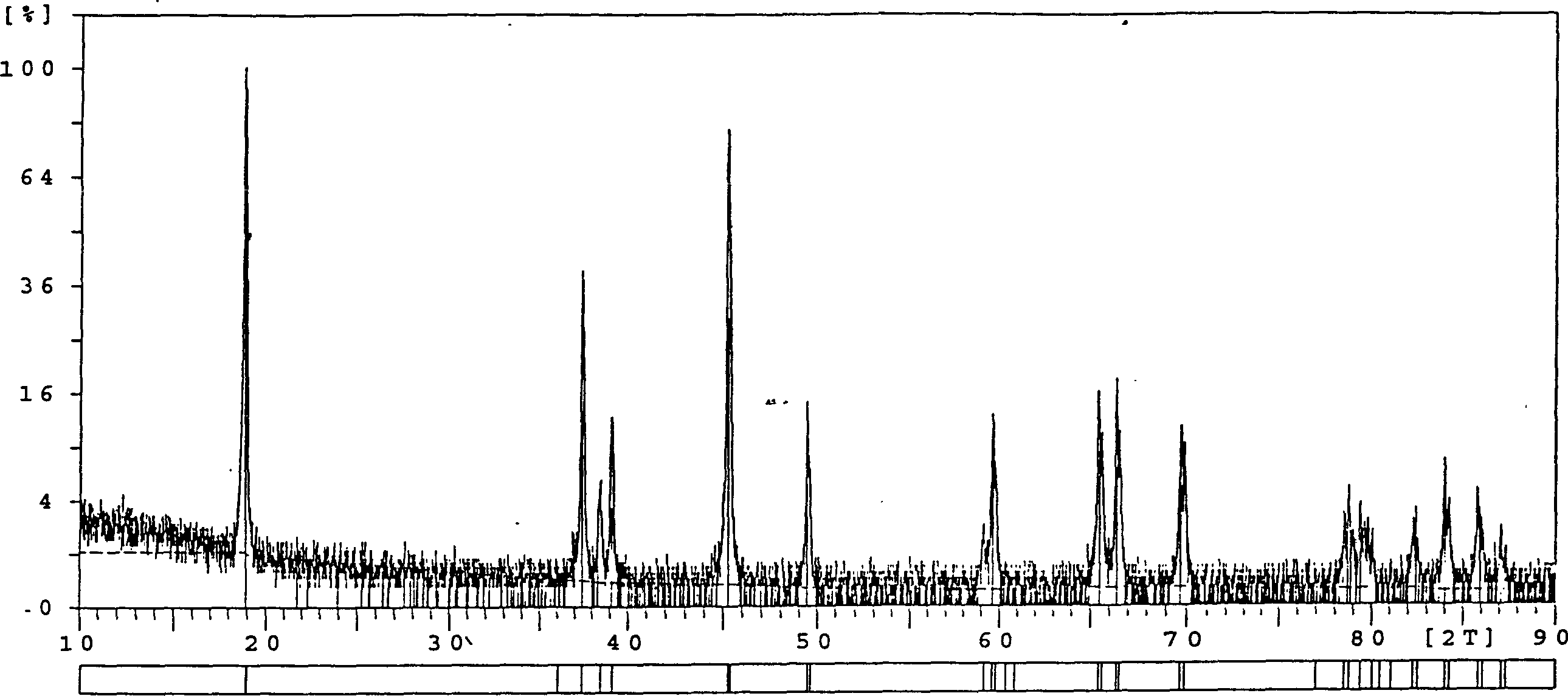
[0024] Use 1.0mol / LCoSO 4 500ml and 1.0mol / L NaOH 1100ml solution are mixed and reacted at room temperature, and an appropriate amount of NaClO is added to generate Co(OH) 3 Precipitate, the precipitate is filtered, washed and dried at 100°C to 150°C to obtain Co(OH) 3 product. 50 g of this product was mixed with LiOH·H 2 O or Li 2 CO 3 Mix according to the molar ratio of Co:Li=1:1.1, add 150-180ml of acetone solution, and keep at room temperature for 2.0-4.0 hours, preferably 3.0-4.0 hours, under the action of stirring and impregnating, the system is in a sol-like state, and the Evaporate and dry at ~110°C, and the homogeneous mixture of lithium cobalt oxides formed is calcined at 850°C for 16 to 18 hours in a muffle furnace to synthesize LiCoO 2 Cathode material, the XRD pattern of this material is shown in figure 1 , the electrical performance test results are shown in Table 1 and figure 2 .
Embodiment 2
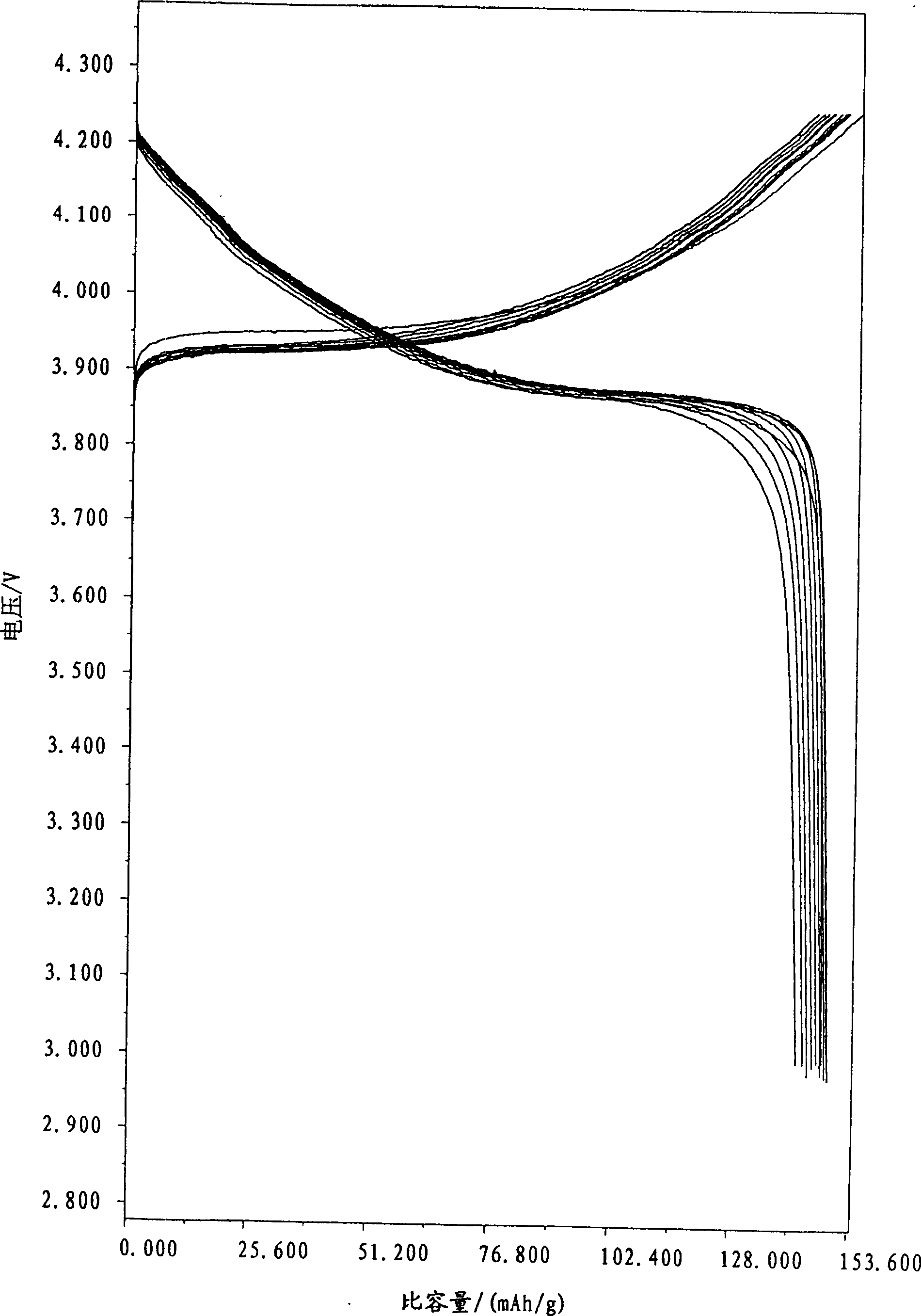
[0026] Use 1.0mol / LCoSO 4 500ml and 1.0mol / L NaOH 1100ml solution are mixed and reacted at room temperature to generate Co(OH) 2 Precipitate, filter and wash the precipitate and dry at 100°C to 150°C to obtain Co(OH) 2 Products, the particle size of which is controlled at 0.5-0.8 μm. This Co(OH) 2 Product 50 g and lithium source LiOH·H 2 O mixes, and ratio is with embodiment 1, and all the other processes are with embodiment 1. The test results of the electrical properties of the samples are shown in Table 1 and Figure 4 , the XRD pattern of this material is shown in image 3 .
Embodiment 3
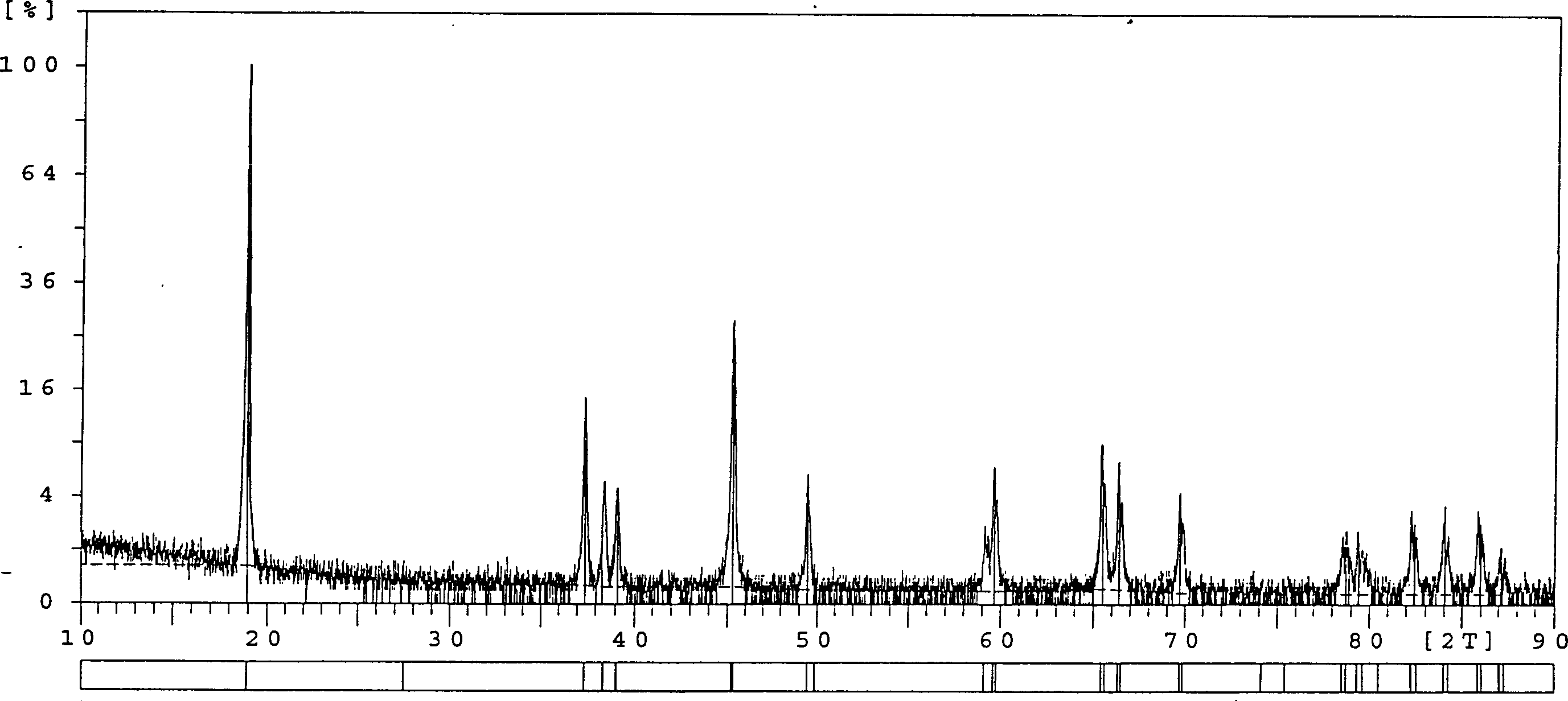
[0028] Will make Co(OH) by embodiment 2 method 2 150g, calcined at 650°C-800°C for 2-6 hours to obtain Co 3 o 4 50g, with high purity (99.9%) Li 2 CO 3 Mix by Co: Li=1.0: 1.02~1.10 mol ratio, add 120~150ml of ethylene glycol aqueous solution of 10%, all the other steps are with embodiment 1, and sample electric performance test result is shown in Table 1 and Image 6 , the XRD pattern of this material is shown in Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com