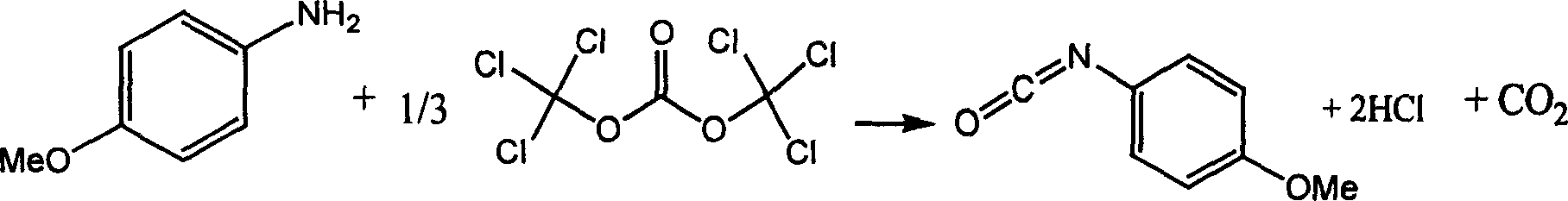
Chemical synthesis of p-methoxyphenyl isocyanate
A technology of methoxybenzene isocyanate and p-methoxyaniline is applied in the fields of pesticides, dyes, and synthetic medicines, and can solve the problems of serious equipment corrosion, long reaction period, and large safety hazards, and achieves low equipment corrosion and low production cost. , the production of safe and reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] P-anisidine: bis(trichloromethyl) carbonate: 1,3-dimethyl-2-imidazolidinone=1:0.35:0.02, the feed amount of toluene is 8 times the mass of p-anis .
[0015] P-Methoxyaniline, 1,3-dimethyl-2-imidazolidinone and toluene are added to the reaction kettle, after stirring, add bis(trichloromethyl) carbonate toluene dropwise within 30 minutes at room temperature At the same time, open the absorption system of the by-product hydrogen chloride to produce industrial hydrochloric acid, and then heat and reflux for 4 hours. After the reaction is completed, the toluene is recovered by atmospheric distillation, and then vacuum distillation. The fraction at 106-108°C is collected under 2000 Pascals. The yield is 95.6 %, content (GC) 99.4%.
Embodiment 2
[0017] P-anisidine: bis(trichloromethyl) carbonate: 1,3-dimethyl-2-imidazolidinone=1:0.35:0.01, the amount of toluene recovered is 8 of the mass of p-anis Times.
[0018] P-methoxyaniline, 1,3-dimethyl-2-imidazolidinone and recycled toluene were added to the reaction kettle, and after stirring uniformly, bis(trichloromethyl) carbonate was added dropwise within 30 minutes at room temperature. Toluene solution, and open the absorption system of the by-product hydrogen chloride to produce industrial hydrochloric acid at the same time, and then the reaction is heated and refluxed for 4 hours. After the reaction is completed, the toluene is recovered by atmospheric distillation, and then vacuum distillation. The fraction at 106-108°C is collected at 2000 Pascals. Yield 96.3%, content (GC) 99.3%.
Embodiment 3
[0020] P-methoxyaniline: bis(trichloromethyl) carbonate: 1,3-dimethyl-2-imidazolidinone = 1:0.50:0.02, the feeding amount of toluene is 8 times the mass of p-anisidine .
[0021] According to the feeding reaction in Example 1, the fraction at 106-108°C was collected at 2000 Pascals, with a yield of 97.0% and a content (GC) of 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com